Key Points
- 1.
Invasive vulvar cancer is a relatively rare tumor, accounting for 4% of all female genital malignant neoplasms.
- 2.
Squamous cell carcinoma of the vulva develops by human papillomavirus- (HPV-) dependent and HPV-independent pathways.
- 3.
Sentinel lymph node biopsy represents the largest innovation in the care of vulvar cancer patients in the past decade.
- 4.
The standard of care is pelvic lymph node irradiation for patients with positive inguinal nodes.
- 5.
Recurrence may be local or distant, and more than 80% will occur in the first 2 years after therapy, demanding initial close follow-up.
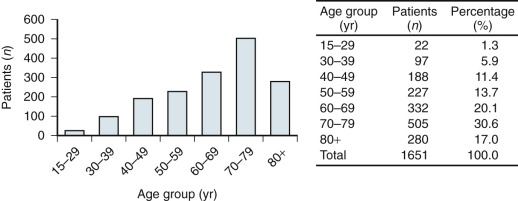
Invasive vulvar cancer is a relatively rare tumor, accounting for 4% of all female genital malignant neoplasms with the American Cancer Society reporting just over 5100 cases resulting in 1080 deaths in 2015 in the United States. With the exception of the rare sarcomas, the peak incidence is in women between 65 and 75 years old ( Fig. 8.1 ); in some series, almost half are 70 years of age or older. Although classically a disease of elderly women, the trend in recent years is an increasing prevalence among younger women, which cannot be accounted for by immune suppression alone. Human papillomavirus (HPV) is a key age-dependent risk factor that causes preinvasive disease in the form of vulvar intraepithelial neoplasia (VIN) that is often associated with a history of tobacco use. HPV-related VIN lesions are rarer in older women, and these malignancies may be associated with chronic vulvar dystrophies, such as lichen sclerosis, although a direct association remains unproven. No race or culture is spared, and gravidity and parity appear unrelated in the pathogenesis of this neoplasm. Vulvar cancer is common in poor and elderly women in most parts of the world, and this has led to the hypothesis that inadequate personal hygiene and medical care are contributing factors in disease development.
Younger patients frequently have early stromal invasion associated with diffuse VIN. Choo found 17 patients younger than age 35 years with invasive carcinoma of the vulva. Of these, eight had microinvasion. Al-Ghamdi and coworkers evaluated 21 patients younger than age 40 years with invasive vulvar cancer and found that most, but not all, had associated HPV. Outcomes in these populations were excellent. Lanneau and colleagues have corroborated these results in 56 women younger than age 45 years with squamous cell carcinoma (SCC) of the vulva. They concluded that vulvar cancer in this population is associated with early-stage disease, HPV, VIN, and smoking.
SCC of the vulva develops by HPV-dependent and HPV-independent pathways. The association between HPV and both preinvasive and invasive urogenital lesions has been well described. Susceptibility of the cervical, vaginal, and vulvar epithelium is referred to as the “field effect,” which is more pronounced in tobacco users and in patients who are immunocompromised, such as those with HIV/AIDS, or organ transplant recipients. The association of condyloma acuminatum with vulvar carcinoma is well known, but no cause and effect relationship has been confirmed as yet. HPV is suspected in the etiology of squamous neoplasia of the vulva, as it is in lesions of the cervix. Bloss and colleagues looked at the clinical and histologic features of vulvar carcinomas analyzed for HPV status. Of 21 invasive carcinomas of the vulva analyzed, 10 were found to contain HPV-16 DNA. Others have confirmed this observation, suggesting that HPV DNA associations with malignant changes of the vulva are similar to those observed elsewhere in the genital tract. Although the incidence of vulvar cancer has remained relatively stable over the past several decades, the incidence of VIN has increased, especially that associated with HPV. Hoang and coworkers contrast these multicentric HPV related precursors with VIN of the differentiated type that occurs in postmenopausal women, often in a background of lichen sclerosis or chronic inflammatory dermatoses associated with a high number of somatic mutations, particularly in TP53. In a recent review, Sznurkowski pointed out the need to better understand the relationship between vulvar cancer and HPV for not only causation but also for developing potential therapeutics.
Many previously reported associated features seen in patients with vulvar cancer, such as diabetes, obesity, hypertension, and arteriosclerosis, may just reflect the increased incidence of these diseases associated with aging.
Invasive Squamous Cell Carcinoma
Histology
The overwhelming majority of all vulvar cancer is squamous in origin. The vulva is covered with skin, and any malignant change that appears elsewhere on the skin can occur in this region. Table 8.1 depicts the incidence of vulvar neoplasia from several collected studies in the literature. The following discussion focuses mainly on SCC because of its preponderance, but as a generalization, the other lesions can be treated similarly, except as noted.

Clinical Presentation and Diagnosis
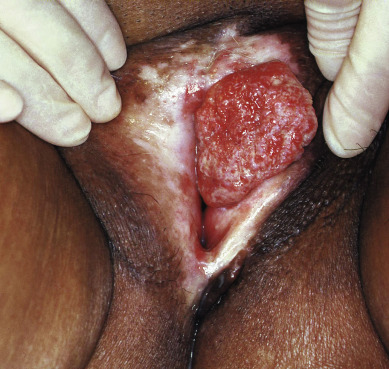
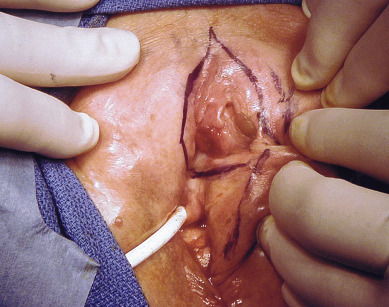
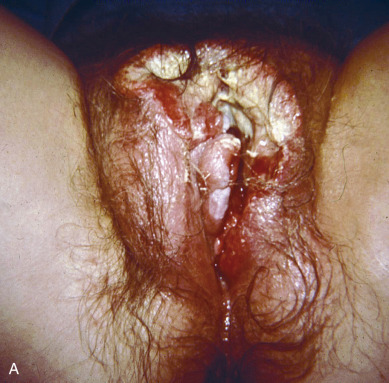
Squamous cell vulvar cancer usually arises from an area of intraepithelial neoplasia that subsequently develops into a small nodule that may break down and ulcerate ( Figs. 8.2 and 8.3 ). On other occasions, small, warty, or cauliflower-like growths evolve, and these may be confused with condyloma acuminatum. Long-term pruritus or a lump or mass on the vulva is present in more than 50% of patients with invasive vulvar cancer ( Table 8.2 ). Biopsy must be done of all suspicious lesions of the vulva, including lumps, ulcers, and pigmented areas, even in the patient not complaining of burning or itching ( Table 8.3 ).
| Sign or Symptom | Incidence (%) |
|---|---|
| Pruritus | 45.0 |
| Mass | 45.0 |
| Pain | 23.0 |
| Bleeding | 14.0 |
| Ulceration | 14.0 |
| Dysuria | 10.0 |
| Discharge | 8.0 |
| Groin mass | 2.5 |
|
A significant delay in diagnosis and appropriate treatment is common. Several series of carcinoma of the vulva report delays of 2 to 16 months after onset of symptoms before medical attention is sought. Further delay can occur when medical treatment of vulvar lesions continues without biopsy for definitive diagnosis.
Fortunately, vulvar cancer is commonly indolent, extends slowly, and metastasizes fairly late. Hence, there is an opportunity for preventing the development of advanced stages of this disease through patient and physician education.
Location and Spread Pattern
Primary disease can appear anywhere on the vulva. Approximately 70% arise primarily on the labia. Disease more commonly occurs on the labia majora; however, it may appear on the labia minora, clitoris, or perineum. The disease is usually localized and well demarcated, although it can occasionally be so extensive that the primary location cannot be determined ( Fig. 8.4 ). Multifocal growth pattern in invasive SCC of the vulva is uncommon, except for the so-called “kissing lesions” that can occur.
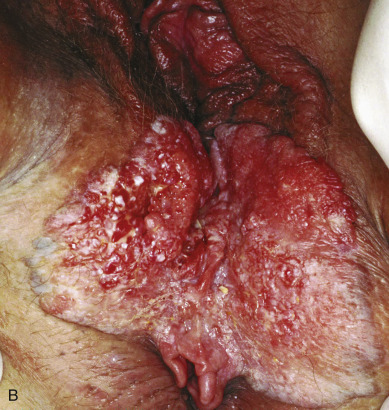
Verrucous carcinoma of the vulva ( Fig. 8.5 ) is a special and unusual variant of SCC that is locally invasive but nonmetastasizing. The lesion, which may involve the cervix, vagina, and the vulva, presents as a warty, fungating, ulcerated mass with a bulky, elevated appearance reminiscent of a benign HPV lesion. Identification of this variant is important because the biologic behavior of the disease influences therapy. Condyloma may initially be diagnosed on microscopic examination, but distinction from ordinary condylomata is aided by the absence of fibrovascular cores within the proliferating papillary masses of tumor. There is usually a uniform lack of malignant features histologically. Adequate material, including underlying stroma for pathologic evaluation, is necessary to differentiate verrucous carcinoma from condyloma. Tumor may invade deeply into the underlying tissue, often requiring extensive surgery, and has a propensity to recur locally. Woodruff noted a lack of lymph node metastases in 27 patients, including a literature review, who were treated with radical vulvectomy and inguinal lymphadenectomy. As a result, a more conservative approach is advocated with wide local excision and tumor-free margins as the therapeutic aim. Lymphadenectomy is of questionable value except when nodes are obviously involved. Historically, it has been thought that radiotherapy is contraindicated because of its ineffectiveness, and reports have indicated that it can incite more aggressive behavior by this tumor.
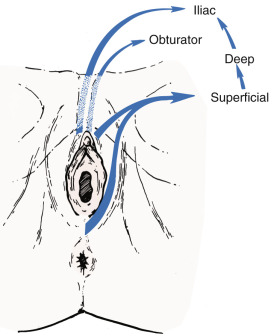
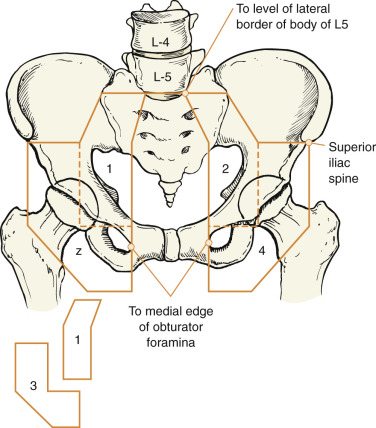
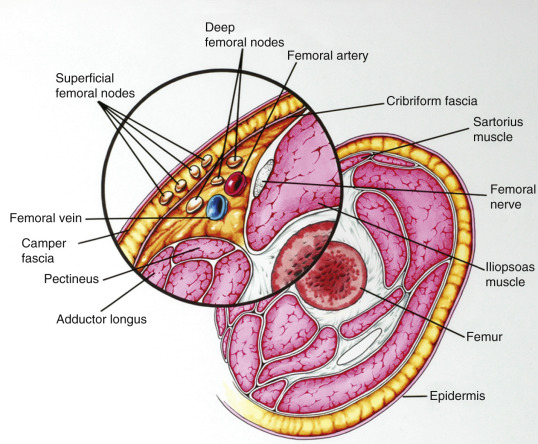
Fundamental to the understanding of therapy for invasive cancer of the vulva is thorough knowledge of the lymphatic drainage of this region. In general, the four histologic types of invasive cancer primarily metastasize via the lymphatic route ( Fig. 8.6 ). Lymphatic drainage of the external genitalia begins with minute papillae that connect to a multilayered meshwork of fine vessels that extend over the entire labium minus, the prepuce of the clitoris, the fourchette, and the vaginal mucosa up to the level of the hymenal ring ( Fig. 8.7 ). Drainage of these lymphatics extends toward the anterior portion of the labium minus, where they emerge into three or four collecting trunks whose course is toward the mons veneris, bypassing the clitoris. Vessels from the prepuce anastomose with these lymphatics. Similarly, vessels from the labium majus proceed anteriorly to the upper part of the vulva and mons veneris, there joining the vessels of the prepuce and labium minus. These lymphatic vessels abruptly change direction, turning laterally, and terminate in ipsilateral or contralateral femoral nodes. Drainage is usually limited initially to the medial upper quadrant of the femoral node group. The nodes are medial to the great saphenous vein above the cribriform fascia and in turn may drain secondarily to the deep femoral group. The next echelon of nodes is the pelvic/iliac nodes.
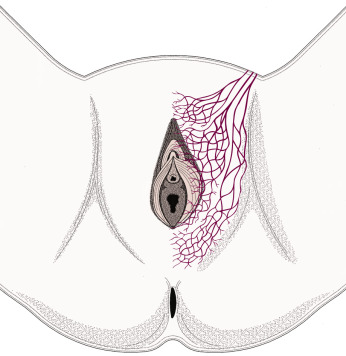
The superficial inguinal lymph nodes are located immediately beneath the integument and Camper fascia, with an average of 8 to 10 in number. Most authors agree that these nodes are the primary drainage for the vulva, thereby containing the sentinel lymph node (SLN) ( Fig. 8.8 ). The deep femoral nodes, which are by classic teaching located beneath the cribriform fascia, are the secondary node recipients and are involved before drainage into the deep pelvic nodes. The Cloquet node , the last node of the deep femoral group, is located just beneath the Poupart ligament. The multilayered meshwork of lymphatics on the vulva itself is always limited to an area medial to the genitocrural fold ( Fig. 8.9 ). Lymphatic drainage of the vulva occurs via a progressive systematic mechanism, and therapy can be planned according to where in the lymphatic chain tumor is present.
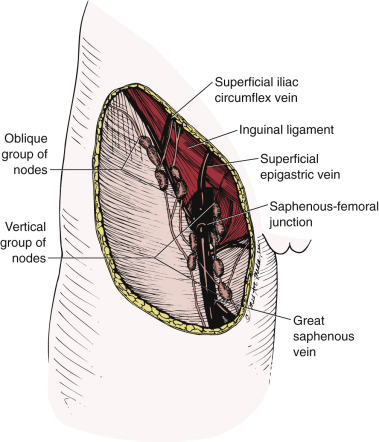
Borgno and colleagues examined 100 inguinal lymphadenectomy specimens at autopsy and demonstrated that the deep femoral nodes are always situated within the openings in the fascia at the fossa ovalis, and no lymph nodes are distal to the lower margin of the fossa ovalis, under the fascia cribrosa. The clinical implication is that a carefully performed deep femoral lymphadenectomy does not require removal of the fascia lata (cribriform fascia) because no lymph nodes are to be found between the femoral vein and artery lateral to the artery or distal to the lower margin of the fossa ovalis beneath the cribriform fascia. They also found that the node of Cloquet or Rosenmüller, which is the uppermost node among the deep femoral lymph nodes, was absent in 54% of dissections. Borgno and colleagues also demonstrated that during surgery, when traction is persistently applied to the lymphovascular fat tissue above the cribriform fascia, all inguinal nodes can be removed. No nodal tissue was found when the cribriform fascia and the fat beneath were submitted separately for pathologic review. Hudson and colleagues confirmed this finding in cadaveric dissections, and Micheletti and coworkers found supporting evidence with embryologic studies.
Although lymphatics draining from the clitoris directly to the deep pelvic lymph nodes are described, their clinical significance appears to be minimal. It is unusual to find a case in which metastasis is present in the pelvic lymph nodes without metastatic disease in the inguinal lymph nodes even when the clitoris is involved. Curry and associates noted clitoral involvement in 58 patients of 191 studied; none had positive deep pelvic nodes without concomitant inguinal node involvement. Similar results were observed in a study of 38 patients, with carcinomas of the clitoris by Ericksson and coworkers, who found also that the deep inguinal or femoral nodes were never positive in the absence of positive superficial inguinal nodes.
The incidence of positive inguinal and pelvic nodes varies considerably, as noted in Table 8.4 . Unfortunately, most of these studies were unstaged, although, in general, the larger the tumor, the greater the propensity for inguinal and pelvic node metastases. Morley noted a 21% incidence of lymph node involvement if there was a T1 lesion (<2 cm in diameter) versus 45% with a T2 lesion (>2 cm but limited to the vulva). Malfetano and colleagues reported the incidence of inguinal node metastases in patients with stage III and stage IV lesions to be 53% and 90%, respectively. Clinical evaluation of the groin is somewhat more accurate than tumor size. Homesley found that approximately 24% of patients with clinically nonsuspicious nodes had positive nodes when dissected, and approximately 75% of patients with suspicious (palpable, fixed, or ulcerated) groins had positive nodes. Table 8.5 demonstrates the association between inguinal lymph node involvement and tumor size, depth of invasion, and clinical examination. Of note, these data did not evaluate laterality or unifocal versus multifocal lesions.
| Series | Cases ( n ) | Positive Groin or Pelvic Nodes (%) | Positive Pelvic Nodes (%) |
|---|---|---|---|
| 374 | 37.0 | — | |
| 191 | 30.0 | 4.7 | |
| 122 | 50.0 | 10.0 | |
| 150 | 24.0 | — | |
| 277 | 29.2 | — | |
| 1553 | 31.0 | — |
| Feature | N | Positive Groin Nodes | % |
|---|---|---|---|
| Tumor Diameter | |||
| ≤2.0 cm | 190 | 36 | 19 |
| >2.0 cm | 390 | 163 | 43 |
| Invasion | |||
| ≤5 mm | 272 | 57 | 21 |
| >5 mm | 286 | 137 | 48 |
| Clinical Examination | |||
| N0-1 | 477 | 114 | 23 |
| N2-3 | 111 | 89 | 80 |
Staging
Many staging systems have been applied to invasive cancer of the vulva. Significant discrepancy exists between clinical and surgical-pathologic evaluation of lymph node status. Iversen demonstrated that overdiagnosis (clinically suspicious but pathologically negative nodes) occurred in 40 of 258 patients (15%). Of the 100 patients with metastasis to the inguinal lymph nodes, lymph node involvement was clinically unsuspected in 36 patients. Patients with “micrometastasis” (lymph node involvement not suspected clinically but positive microscopically) had a significantly better survival rate than did those with gross metastasis. As a result of these repeated findings, it was suggested that staging be based on surgical-pathologic evaluation instead of clinical evaluation alone. Federation of International Gynecologists and Obstetricians (FIGO) staging of vulvar cancer dates back to 1969. In 1988, FIGO replaced the clinical staging system with a surgical-pathologic, or tumor–node–metastasis (TNM), classification system. The most recent 2009 FIGO staging for carcinoma of the vulva ( Table 8.6 ) further characterizes the number and type of lymph node metastases. Previous studies noted decreased survival among individuals with bilateral lymph node metastasis; thus, laterality was incorporated into the staging system. However, recent studies have demonstrated that the number of lymph node metastases and extracapsular spread are predictive of survival regardless of laterality. It is important to consider that many reports in the literature use older staging systems, and thus data must be evaluated within the context of the staging system used.
| Stage | Description |
|---|---|
| I | Tumor confined to the vulva |
| IA | Lesions ≤2 cm in size confined to the vulva or perineum and with stromal invasion ≤1.0 mm * ; no nodal metastasis |
| IB | Lesions >2 cm in size confined to the vulva or perineum with stromal invasion greater than 1.0 mm * ; no nodal metastasis |
| II | Tumor of any size with extension to adjacent perineal structures (1/3 lower urethra, 1/3 lower vagina, anus); no nodal metastasis |
| III | Tumor of any size with or without extension to adjacent perineal structures (1/3 lower urethra, 1/3 lower vagina, anus) with positive inguinofemoral lymph nodes |
| IIIA |
|
| |
| IIIB |
|
| |
| IIIC | With positive nodes with extracapsular spread |
| IV | Tumor invades other regional (2/3 upper urethra, 2/3 upper vagina) or distant structures |
| IVA | Tumor invades any of the following: |
| |
| |
| IVB | Any distant metastasis, including pelvic lymph nodes |
* The depth of invasion is defined as the measurement of the tumor from the epithelial–stromal junction of the adjacent most superficial dermal papilla to the deepest point of invasion.
Donaldson and colleagues demonstrated that lesion size predicted incidence of lymph node metastasis (19% metastasis for lesion <3 cm and 72% for lesion >3 cm). Likewise, tumor grade correlated with node metastasis (one-third of well-differentiated tumors had metastasis compared with 75% for poorly differentiated lesions). Of 38 patients, 11 (29%) had node involvement if invasion of the primary lesion was 5 mm or smaller compared with 17 of 28 (61%) if invasion was larger than 5 mm. If the tumor did not involve lymphatic or vascular spaces, only 2 of 33 (6%) had positive nodes, but 26 of 33 patients (79%) with lymphatic or vascular space involvement had metastasis to the regional lymph nodes. None of 25 patients with lesions invading less than 5 mm and without lymphatic or vascular space involvement had lymph node metastasis.
Tabbaa and associates reported the largest cohort study to evaluate the effect and prognostic performance of the 2009 FIGO staging. They reported FIGO stage I, II, III, and IV 5-year overall survival (OS) rates of 84%, 75%, 48%, and 9%, respectively. The 5-year OS rate was 85% for patients without LN metastasis, and for patients with three or more lymph node metastases, the 5-year OS rate was 30%. They surmised that bilateral nodal disease does not appear to impact cause-specific survival, justifying its omission from the 2009 staging system, and that separating node-positive stage III from node-negative stage II cases appears justified.
Sentinel Lymph Node Biopsy
Sentinel lymph node biopsy (SLNB) represents the largest innovation in the care of patients with vulvar cancer in the past decade. The use of the SLN technique in the evaluation of vulvar cancer was first described in the late 1970s based initially on the experience in penile cancers followed by the successes reported in breast cancer and melanoma. The past 20 years have witnessed a myriad of reports featuring SLN techniques in the management of gynecologic cancers, including vulvar cancer, that have provided experienced clinicians with a standard of care option for surgical lymph node management in appropriately selected patients. The concept of SLNs depends on reliable prediction of a tracer substance identifying the primary nodal drainage of the tumor. The assumption is that if the SLN is negative for malignancy then the other nodes will be negative thus precluding the need for full nodal dissection and thereby significantly reducing the procedural related short-term infection and wound breakdown and long-term edema associated with inguinal femoral lymph node dissection (IFLND).
Only approximately 30% of early stage vulvar cancers have positive nodes with IFLND, which thus exposes a large number of women to ostensibly an unnecessary morbid procedure wherein up to two-thirds of patients experience lymphedema. Therefore, reducing the radicality of groin lymphadenectomy by SLNB merits investigation, validation, and implementation as a reliable alternative to full nodal dissection that requires balancing the slightly higher groin recurrence rates of SLNB versus the significantly greater morbidity of IFLND.
Concerns raised in adopting SLNB as the standard of care include first, the reliability of SLN detection. How feasible is this technique in terms of how often does one detect an SLN, and which technique should be used in terms of injection site and tracer selection? Second, what is the reliability of a negative SLN to predict that all the other inguinal nodes are likewise negative for malignancy? In other words, what is the potential for a false-negative result, whereby a SLNB does not demonstrate metastases but additional groin dissection discovers lymphatic spread? Finally, what is the groin recurrence rate? This question is critical because untreated groin metastases will result in groin recurrences that are most often fatal. It is important to recall that the gold standard for the rate of groin relapse in a node-negative groin is 0.3% based on Homesley et al.’s Gynecologic Oncology Group (GOG) experience. The data for detection rate, sensitivity, or false-negative results can be considered from the perspective of each groin, viewed as a separate entity, or from the patient’s perspective whereby the outcomes of SLNB are considered for both groins as a unit.
Another concept to consider is the role of nodal ultrastaging with very fine sectioning and use of immunohistochemistry (IHC) as an adjunct to conventional staining, in which the SLN is subjected to far greater scrutiny in terms of pathologic sectioning than nodes removed via traditional IFLND. Nodal yield from traditional IFLND is much greater in number, and these nodes are not usually subjected to these labor-intensive techniques because of time and resource constraints. Also, a statistical concept known as the false-negative predictive value (FNPV), defined as (1- Negative predictive value), needs to be understood to properly consider the burgeoning SLNB literature in the management of vulvar cancer.
Several small studies were instrumental in validating the feasibility of SLNB, with the original lymphatic mapping data being published by Levenbach and colleagues in 1994. Recent studies have continued to demonstrate improved outcomes for SLNB over time. Initial mapping studies of SLNB in vulvar cancer evaluated 21 patients with isosulfan blue dye only, and an SLN was identified in only 86% of patients and 66% of groins. Addition of lymphoscintigraphy (LSG) with radiolabeled technetium significantly improved the SLN detection rate. A collective review of the literature in 2002 noted a detection rate of 92% with the combined technique. Frumovitz and colleagues reported on 52 women who underwent SLNB 1993 and 1999; 14 had a recurrence. Eight of the recurrences (15.4%) were on the vulva, three (5.8%) in the groin, and three (5.8%) distant. The pattern of recurrence is similar to that seen with standard approaches, although the rate of relapse in the groin appears high.
One of the first large prospective multicenter observational trials to assess SLNB was the GROINSS-V I study reported by Van der Zee and associates that included 403 assessable patients using the combined technique. Eligible patients had T1 or T2 lesions smaller than 4 cm, invasion greater than 1 mm, and clinically nonsuspicious inguinofemoral lymph nodes. SLNB was performed, and if the SLN result was negative, then an IFLN was not performed; only when SLN metastases were identified was an IFLND performed either at time of surgery based on frozen section or subsequently based on final pathologic analysis of the SLN. A total of 623 groins in 403 patients were evaluated. The authors selected 276 selected patients with unifocal vulvar disease and a negative SLN to describe in detail, and the 3-year survival rate was 97%, with a mean follow-up of 35 months. Adjuvant radiotherapy was administered to the groin and pelvis when more than one lymph node demonstrated metastasis or extracapsular nodal tumor growth was identified. Those who underwent SLNB had a FNPV of 2.9% with a false-negative rate of 6%. The groin recurrence rate for those with negative SLNs was 2.3%. Patients with unifocal lesions had only a 2% recurrence rate compared with 12% for those with multifocal lesions on the vulva. Lymphedema was noted in 2% for SLNB versus 25% in those with positive SLN who underwent full IFLND. Wound breakdown and recurrent cellulitis occurred in 12% and 0.4% versus 34% and 16% for SLNB versus completion IFLND procedures respectively. Ultrastaging with three sections per mm was performed only on SLNs that were negative on routine histopathologic examination. Ultrastaging significantly increased detection of nodal metastases. Routine pathologic examination detected 95 (58%) of the 163 groins with metastatic SLNs, and ultrastaging detected the remaining 68 (42%) groins demonstrating metastatic SLNs.
A subset analysis of the GROINSS-V I study conducted by Oonk and colleagues demonstrated that the risk of non-SLN metastases increased with increasing size of SLN metastasis. Also, the risk of non-SLN metastases was higher when the SLN was found to be positive with routine pathology than with ultrastaging. The 5-year disease-specific survival rates were 97% for women with isolated tumor cells in the SLN, 88% for those with SLN metastases smaller than 2 mm, 70% for those with metastases measuring 2 to 5 mm, and 69% for those with metastases larger than 5 mm. No cutoff provided complete assurance that the non-SLNs would be free of metastases thus given the high risk of mortality if such nodes are missed; additional groin treatment remains the current recommendation for all patients with SLN involvement.
GOG 173 reported by Levenback and associates included 452 women who underwent SLNB followed by IFLND irrespective of SLNB findings. Eligibility criteria included SCC confined clinically to the vulva with at least 1 mm of invasion and tumor size up to 6 cm in greatest diameter. Patients with groin nodes that were clinically suggestive of cancer were excluded. Every woman underwent intraoperative lymphatic mapping using isosulfan blue dye; however, 2 years into the study, the protocol was amended to require preoperative LSG and intraoperative radiolocalization, which previously had been optional. Nodes were subjected to ultrastaging by serially sectioning them into 3-mm blocks with at least two sections 40 microns apart per block. The use of frozen section was discouraged. If routine hematoxylin and eosin staining was negative for metastatic disease on the first slide, IHC cytokeratin staining was performed on the second slide. A total of 772 groin dissections were performed (320 = bilateral and 132 = unilateral) in 452 women of whom 418 exhibited at least one SLN. Incidence of node positivity among women with at least one SLN identified was 32%. Of the 132 women with positive nodes, 11 had false-negative findings on SLNB (8.3%; 90% confidence interval [CI], 4.7%–13.4%); thus, the sensitivity was 92%. Importantly, this study showed that the false-negative rate for SLNB increased significantly when tumor size was 4 cm or larger (7.4% vs. 2% for tumors <4 cm). They also demonstrated a favorable FNPV of 3.7%, which was the primary statistical endpoint of this trial. This FNPV is comparable to that observed in breast cancer, but salvage rates for that disease are more favorable. This study highlighted the role of pathologic analysis as 23% of the positive nodes were missed on conventional staining and required IHC for detection of cancer. SLN ultrastaging is also important via step sectioning, which significantly increases the chance of finding metastases, according to Puig-Tintore et al.
Coleman et al. published a subset analysis of patients from GOG 173 that examined the importance of the location of the primary tumor relative to the midline and the role of LSG, which was optional in this study. A total of 234 patients were eligible with 64 having lateral lesions (>2 cm from midline) and thereby undergoing only unilateral IFLND after SLNB; the 105 women with midline and 65 with near-midline lesions (<2 cm distance from midline) underwent bilateral completion IFLND. Bilateral groin drainage using LSG was discovered in 22% of lateral, 58% of near-midline, and 70% of midline tumors. At mapping, no SLNs were found in contralateral groins among patients with near-midline and midline tumors who had unilateral-only LSGs, yet groin metastases were found in four of 32 patients with midline tumors undergoing contralateral dissection; none were found in 27 patients with near-midline tumors. They concluded that the likelihood of detectable bilateral drainage using preoperative LSG decreases as a function of distance from midline. Patients with near-midline primary tumors exhibiting unilateral drainage on LSG may safely undergo unilateral SLN. Further validation of this concept is indicated.
A Cochrane review of SLNB in vulvar cancer management was reported by Lawrie et al. that assessed the diagnostic test accuracy of various techniques using traceable agents for SLNB to diagnose groin lymph node metastasis in women with FIGO stage IB or higher vulvar cancer. A total of 34 studies evaluating 1614 women and approximately 2396 groins were included in the analysis. Use of blue dye only provided a sensitivity of 0.94 (68 women; 95% CI 0.69–0.99); for mixed tracers, sensitivity was 0.91 (679 women; 95% CI 0.71–0.98); and for technetium only, it was 0.93 (149 women; 95% CI 0.89–0.96). Sensitivity for combined tests was 0.95 (390 women; 95% CI 0.89–0.97). Negative predictive values (NPVs) for all index tests exceeded 95%. Mean detection rate for blue dye alone was 82%, compared with 95%, 96%, and 98% for mixed tests, technetium only, and combined tests, respectively. It was estimated that for 100 women hypothetically undergoing SLNB assuming groin metastases prevalence of 30% for the combined or technetium only tests, one and two women with groin metastases might be “missed,” and for mixed tests, three women with groin metastases would occur as false-negative results. This review concluded that there are only minor differences in diagnostic test accuracy between the technetium and combined tests. Combined testing may reduce the number of false-negative results compared with technetium alone. It must be recognized that there is a learning curve to successful SLNB, and combined tests may further increase detection for less experienced surgeons. Blue dye alone may be associated with more FNs compared with tests that include technetium. They further concluded that SLNB would reduce the need for full IFLND by 70% in patients with early vulvar cancer, but long-term survival data are needed.
A systematic review was presented by Meads et al. to assess the accuracy of SLNB with technetium 99 and/or blue dye–enhanced LSG. A total of 29 studies that included 1779 women were analyzed. Most studies used technetium-99 combined with blue dye. Pooling when appropriate revealed mean SLN detection rates of 94% for technetium, 69% for blue dye alone, and 98% for both. SLNB revealed a pooled sensitivity of 95% (95% CI 92%–98%) with an NPV of 97.9% in studies using technetium with blue dye, ultrastaging, and IHC with inguinal lymphadenectomy as reference. Less morbidity was reported for those undergoing SLNB. The authors concluded that SLNB should be done in carefully selected patients with technetium and blue dye with ultrastaging and IHC. They further concluded that patients must make an informed choice between the slightly higher groin recurrence rates of SLNB versus the higher morbidity with full lymphadenectomy.
Another systematic review by Hussanzade et al. included 49 studies and reported a false-negative rate by patient and groin perspectives of 8%. Pooled NPVs were 97% for patient- and groin-based analyses. They pointed out that those with palpable groin nodes had lower detection rates and sensitivity with SLNB. They concluded that SLNB with radioactive tracer plus blue dye was accurate in appropriately selected patients with exclusion of those with clinically suspicious inguinal nodes. They also raised the concern of an increased FN rate for midline tumors.
Slomovitz and colleagues performed a review recently that declared SLNB as the standard of care for early stage vulvar cancer secondary to lymphedema rates of 30% to 70% after traditional IFLND combined with the robust prospective data from GOG 173 and the GROINSS-V I studies that confirmed the feasibility and utility of SLNB with similar false-negative rates of 6% for tumors smaller than 4 cm. Important differences between these studies include the fact that GROINSS-V had smaller tumors (T1 or T2 <4 cm) versus GOG 173 in which T1 and T2 tumors measuring 2 to 6 cm were included. Mapping techniques also differed because GROINSS-V required both dye and tracer as well as a minimum threshold of 10 cases for skill verification versus blue dye only was required initially with no skill verification in GOG 173. The authors concluded that the groin relapse rate attributable to a false-negative SLNB for primary vulvar tumors smaller than 4 cm was less than 3%.
Covens and associates convened a working group panel that performed an additional systematic review with meta-analysis whereby they reported a per groin detection rate for SLNB using radiocolloid tracer and blue dye of 87% and a false-negative rate of 6.4%. They recommended SLNB for women with unifocal tumors smaller than 4 cm with clinically nonsuspicious groin nodes, provided that conditions of specific infrastructure and SLN experience and volume are met. The groin recurrence rates are 6.6% for superficial IFLND and 1.4% for complete IFLD versus 3.4% for SLNB. Expert panel recommendations from this paper included using radiocolloid with blue dye and not using blue dye alone secondary to low detection rates. Four quadrant intradermal injections into normal tissue at the margins of the tumor were recommended. Near-infrared tracers (eg, indocyanine green [ICG]) as well as the role of frozen sections require further study. Radiocolloids can be injected 30 min to 24 hours before surgery, depending on the size of the radiocolloid, and manufacturer directions, and blue dye should be injected in the same location as the radiocolloid after induction of anesthesia. A node that has more than five times the background radioactivity should be used to identify an SLN. Pathologically, the panel advocated for SLN ultrastaging by serially sectioning into 3-mm blocks and at least two sections 40 µm apart from each block. If routine hematoxylin and eosin staining is negative for metastatic disease on the first slide, IHC cytokeratin staining should be performed subsequently. Finally, the panel endorsed omission of IFLND in the contralateral side to a positive node when the SLN has been negative in that contralateral side, although they acknowledged that data are limited.
Some issues with SLNB remain less clear, but data are emerging. What is the risk of contralateral non-SLN metastasis in patients with unilaterally positive SLNs? Woelber and colleagues addressed this question in 140 patients, 124 with bilateral and 16 with unilateral SLN dissection. A median number of two SLNs with a range of one to seven per groin were dissected. Of 33 patients with unilaterally positive SLNs, 28 (85%) underwent complete bilateral IFLND despite a contralateral negative SLN, and none demonstrated contralateral non-SLN metastasis. Of the remaining five patients, three received postoperative radiotherapy to the groins. One woman (3.6 %), however, did develop groin recurrence in the initially SLN-negative, fully dissected groin after 19 months. These data support the omission of contralateral IFLND if negative contralateral SLN is found, but clearly further study is needed before altering current guidelines that mostly advocate for bilateral IFLND in these circumstances because these numbers are small and possibly confounded by groin irradiation.
Another unanswered question with SLNB heretofore has been the effect on long-term survival. This issue was recently addressed by te Grootenhuis and associates, who reported the long-term follow-up from the GROINSS-V I study with a median follow-up period of 105 months. The overall local vulvar recurrence rates were 27% at 5 years and 40% at 10 years after primary treatment. These recurrence rates are high considering that these were patients with unifocal tumors smaller than 4 cm. Notably, 89% of patients underwent wide excision versus only 10% who underwent radical vulvectomy for the primary tumor. For SLNB-negative patients, recurrence rates were 25% and 36% at 5 and 10 years, respectively, but SLNB positive patients demonstrated 33% and 46% recurrence rates at 5 and 10 years, respectively. A total of 39 (15%) SLNB-negative patients underwent IFLND secondary to a local recurrence. The isolated groin recurrence rates were 2.5% for SLNB-negative patients and 8% for SLNB-positive patients at 5 years of follow-up. The overall 10-year disease-specific-survival rates were 91% for SLNB-negative patients versus 65% for SLNB-positive patients. For all patients, 10-year disease-specific survival rate decreased from 90% for patients without a local recurrence to 69% for those with a local recurrence.
Additional questions with SLNB remain including optimal imaging techniques such as ultrasonography, computed tomography (CT), magnetic resonance imaging, positron emission tomography/CT, and most recently, single-photon emission computed tomography (SPECT) preoperatively, that would best exclude clinically suspicious nodes that are thought to considerably increase the FN rate for SLNB. Nodes that are totally replaced by tumor may not be identified by mapping because of blockage of lymphatic channels by tumor, thereby resulting in a false-negative result. The role of prior local recurrences as well as prior SLNB also are poorly understood in terms of the accuracy of repeating SLNB. Furthermore, the clinical consequences of micrometastases are unclear. As SLN metastasis size increases, the probability of non-SLN metastases increases, which correlates with decreasing survival. SNLB appears to be cost effective in separate reports (McCann et al. and Erickson et al.); nonetheless, Sutton and associates performed a model-based economic analysis that clearly showed that IFLND was most cost effective and dominated all other strategies considering 2-year OS, but when morbidity-free outcomes were considered, then SLNB using a combined identification technique with ultrastaging and IHC was superior.
Future directions in optimizing SLNB will include development of novel tracers and dyes such as fluorescent dyes (eg, ICG) for enhanced SNL identification. Additionally, ongoing trials GROINSS-V-II and GOG270 will address the potential role of adjuvant groin irradiation as a substitute for completion IFLND in those with positive SLNB. It is noteworthy that this trial suspended accrual in 2010 because of higher than allowable groin recurrences in the cohort of patients with positive SLNB who received adjuvant radiotherapy. At the time of suspension, 10 groin recurrences were identified in 82 such patients. Extensive analysis of these cases revealed that tumor metastases size (≥2 mm) and extranodal extension were associated with a statistically higher rate of groin recurrence. The trial was reopened for accrual after amending the eligibility criteria. Only those with micrometastases remain eligible for radiation; those with macrometastases are to undergo IFLND.
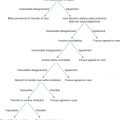
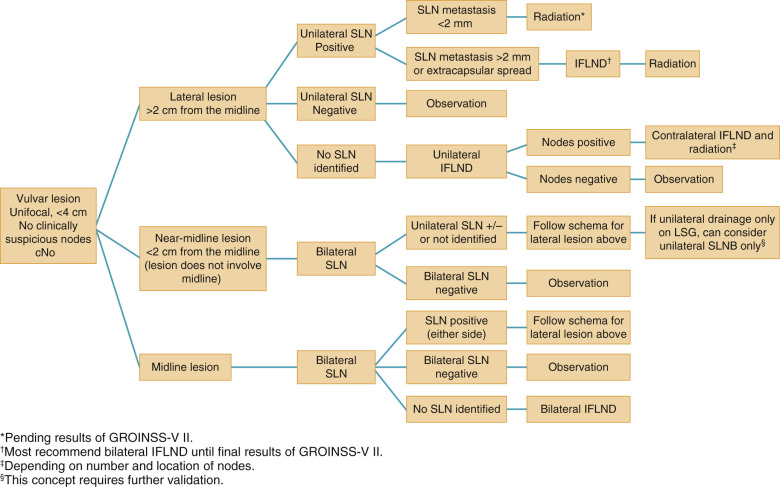
In summarizing, if an SLN is not identified and in any case in which the mapping procedure appears inadequate, SNLB should be abandoned in favor of complete IFLND. Patient selection is crucial to success. Patients should have a negative metastatic workup. Table 8.7 summarizes factors that affect the success of SNLNB. Ideal candidates have unifocal T 1 or T 2 primary tumors smaller than 4 cm in diameter and clinically nonsuspicious groin nodes. SNLB performance characteristics from select studies are summarized in Table 8.8 . The standard has been that if a positive SLN is found on one side, then a complete groin dissection should be done bilaterally; however, emerging evidence supports omission of full contralateral IFLND if the contralateral SLN is negative. Fig. 8.10 proposes a potential management algorithm for SLNB. Clearly, these management schemes require individualization as well as constant vigilance of emerging literature that may alter recommendations.
|
| Author, Study, Year | Patients ( n ) | SLN Detection Rate (%) | Sensitivity (%) | NPV (%) |
|---|---|---|---|---|
| 403 | NR * | 98 | 97 | |
| 452 | 93% | 92 | 96 † 98 ‡ | |
| Lawrie, review, 2014 | 1614 (aggregated) | 98 | 95 | 98 |
| 1779 (aggregated) | 97.7 | 95 | 97.9 | |
| Hussanzade, review, 2013 | 981 (aggregated) | 94.4 | 92 | 98 |
| Covens, review, 2015 | 1087 (aggregated) | 87 | 94 | NR |
Sentinel nodes
Management
Overall, management of invasive vulvar cancer has witnessed an evolution from large en bloc resection associated with high morbidity to more conservative approaches, such as radical local resection, separate groin incisions, and now SLNB to minimize complications such as wound breakdown and infection, lymphocele formation, and chronic lymphedema. Way originally reported improved survival in carcinoma of the vulva by use of the en bloc dissection of radical vulvectomy plus inguinal and pelvic lymphadenectomy, which subsequently became the mainstay of treatment in vulvar cancer. The corrected 5-year survival rate for stage I and stage II disease has been reported by many authors to be approximately 90%. This so-called “longhorn” or “butterfly” en bloc resection has evolved into a significantly more tailored approach depending on size and laterality of the lesion, resulting in similar cure rates with lower morbidity. Modified radical vulvectomy is the current approach to resect the primary lesion with a 2-cm margin, sparing a significant amount of normal tissue, especially with smaller lesions. Lymph nodes are resected via separate incisions. Well-lateralized lesions, defined as more than 1 cm from the midline, can be managed with local radical excision or modified radical vulvectomy and ipsilateral inguinofemoral lymph node dissection (LND). Homesley and coworkers presented the GOG experience with such lesions ( Table 8.9 ) and confirmed the low incidence of contralateral node involvement, making ipsilateral inguinal lymphadenectomy a rational approach. However, central lesions must be evaluated with bilateral LND. This is based on the lymphatic drainage of the vulva confirmed by anatomic studies demonstrating contralateral lymphatic drainage of lesions near the anus and clitoris, thus warranting the evaluation of both groins in central lesions.
| Tumor Thickness (mm) | Ipsilateral Positive Only | Contralateral Positive Only | Bilateral Positive | Total | Patients ( n ) |
|---|---|---|---|---|---|
| <2 | 6.8 | 0.0 | 0.0 | 6.8 | 59 |
| 3–5 | 20.4 | 1.9 | 2.8 | 25.0 | 108 |
| 6-10 | 28.8 | 3.8 | 11.3 | 43.8 | 80 |
| >11 | 36.7 | 6.7 | 6.7 | 50.0 | 30 |
| Total | 21.7 | 2.5 | 5.1 | 29.2 | 277 |
Adjuvant therapy is based on surgicopathologic findings after radical vulvectomy and evaluation of appropriate lymph nodes. Historically, if metastasis was noted in the groin nodes, pelvic LND was performed. This practice has been largely abandoned in favor of pelvic radiation therapy to treat the next nodal chain when groin nodes are involved. The pelvic nodes are essentially never involved with metastatic disease when the inguinal nodes are uninvolved. A study by Curry et al. showed that of 191 patients, only nine (5%) had positive pelvic nodes, and all nine patients also had metastatic disease in the groin nodes. The GOG (Homesley et al.) performed a prospective randomized study in patients with vulvar carcinoma who had positive groin nodes; 114 patients with positive inguinal nodes were randomly assigned to receive radiotherapy versus ipsilateral pelvic node dissection. The group treated with radiotherapy had a 68% relative 2-year survival rate, and the group undergoing pelvic node dissection had a 54% relative 2-year survival rate. Thus the standard of care became pelvic lymph node irradiation ( Fig. 8.11 ) for patients with positive inguinal nodes.
A randomized GOG trial by Stehman and colleagues attempted to evaluate groin irradiation versus groin dissection for patients with N0/N1 nodes. This study was closed prematurely when interim monitoring demonstrated an excess groin failure rate in the radiation arm. Although this study’s radiation prescription has been criticized, a Cochrane Database Systematic Review ( ) concluded that follow-up studies do not support better disease control with radiation of the intact groin and that LND continues to be the cornerstone of therapy. There may still be some role for radiation therapy, especially in patients who are not surgical candidates, although the risks of groin failure and hip fracture must be considered ( ).
Boronow also emphasized the possible role of primary radiation therapy in vulvar and vaginal cancers. His report dealt mostly with advanced disease involving vaginal tissues, necessitating an exenterative procedure if a primary surgical approach was used. As an alternative, he recommended surgical extirpation of the lymph nodes with a combination of external and interstitial irradiation for control of the central lesion. In a small, highly individualized series, this approach appeared promising. Similar reports by Fairey and associates and Hacker and colleagues substantiated this. Although neoadjuvant radiation has gained acceptance in this setting, significant challenges remain. Low anterior and posterior fields must be used, resulting in intense exposure of the vulvar skin because the axis of the x-ray beam runs parallel to (and often within) the skin and mucous membrane. Vulvitis may result, and interruption of therapy is often necessary because of the patient’s discomfort. Similarly, radiating enlarged, grossly positive inguinal nodes becomes technically difficult. Surgical removal of enlarged nodes with subsequent radiation therapy to the area has been our preference. Requisite preoperative doses of 45 to 50 Gy to either the groin or vulvar areas significantly complicates any subsequent surgery because wound healing is impaired. Delaying surgical intervention for 4 to 6 weeks after radiation may reduce these risks.
Neoadjuvant concurrent chemoradiation therapy to provide optimal reduction in the size of the central lesion has also been evaluated. Russell and coworkers described 25 women with locoregionally advanced squamous cancer of the vulva. All patients received external-beam radiation and synchronous radiopotentiating chemotherapy. Complete clinical response was obtained in 16 of 18 previously untreated patients and in four of seven patients with recurrent disease. Concurrent chemoradiation with cisplatin and 5-fluorouracil (5-FU) has been prospectively evaluated by the GOG and found to be highly effective. Moore and colleagues reported on 73 patients with T3 or T4 tumors who would have required ultraradical surgery to clear disease. Resection was accomplished after chemoradiation in 69 of 71, and only three patients required urinary or fecal diversion. Montana and colleagues reported the companion trial for patients with unresectable groin nodes. Many of these poor-prognosis patients experienced progression or intercurrent death during chemoradiation. Still, 38 of 40 who completed treatment had resectable disease, and 15 of 37 had negative lymph nodes.
It has been suggested that in selected stage IV carcinomas of the vulva, ultra-radical surgery may be applicable. Cavanagh and Shepherd, in a review of their data and the literature, identified 53 patients since 1973 who were treated with exenteration and radical vulvectomy. Most of the patients were young, and 47% were alive without recurrence at 5 years. In their series, Cavanagh and Shepherd found all survivors to have negative pelvic lymph nodes.
Technique of Radical Vulvectomy
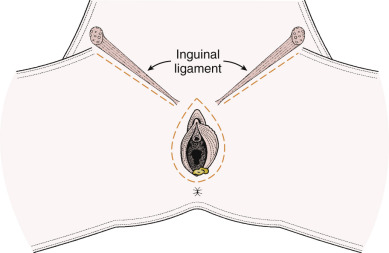
The goal of radical vulvectomy is to remove the primary lesion to the depth of the perineal fascia with a 2-cm circumferential margin. Ipsilateral or bilateral inguinofemoral lymph nodes are harvested based on the size and laterality of the lesion, as discussed previously. The single-incision technique is described in Fig. 8.12 and can be modified to incorporate one or both groins. Today, most limit the skin incision to the inguinal area, thereby preserving the bridge of skin between the inguinal and vulvar incisions (three-incision technique, Fig. 8.13 ). All tissue is removed from the inguinal lymph node bundles. An 8-cm incision is made parallel to the inguinal ligament two fingerbreadths (4 cm) beneath the inguinal ligament and two fingerbreadths (4 cm) lateral to the pubic tubercle ( Fig. 8.14 ). The incision is carried down through the Camper fascia, and at this point, skin flaps are bluntly and sharply dissected superiorly and inferiorly, allowing access to the fat pad containing the superficial nodes. The SLNs are located in the fatty layer of tissue beneath the Camper fascia, in part anterior to the cribriform plate and protruding from beneath the fascia lata ( Fig. 8.15 ). The dissection should be carried superiorly to the inguinal ligament and inferiorly to a point approximately 2 cm proximal to the opening of the Hunter canal. The dissection should be carried laterally to the sartorius muscle and medially to the adductor longus muscle fascia ( Fig. 8.16 ). Blunt dissection facilitates identification of the cribriform fascia, which is most easily identified just below the inguinal ligament or in the area of the saphenous opening. The cribriform fascia unites with the fascia lata, and thus is contiguous with the fascia on the surface of the adductor longus and sartorius muscles; this may facilitate its identification. The portion of the fascia covering the femoral triangle is perforated by the saphenous vein, by lymph nodes of the vertical set, and by numerous blood and lymphatic vessels, hence the name cribriform fascia . If the dissection is carried out properly, the adventitia of the femoral vessels should not be clearly seen except through the vessel openings mentioned earlier.

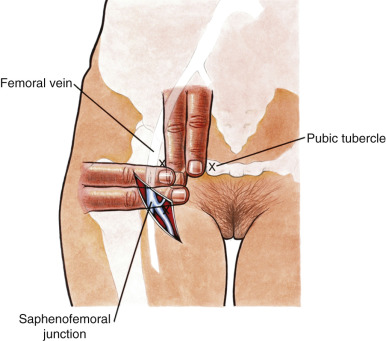
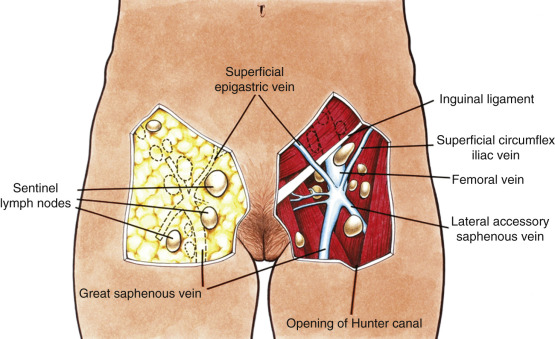
Bell showed that a complete inguinal dissection could be achieved while leaving the fascia intact. He and his co-investigators retrieved a mean of 10 nodes per groin without removing the cribriform fascia. Borgno and colleagues demonstrated that the deep inguinal or femoral nodes are exposed in the fossa ovalis and other openings of the cribriform fascia, allowing access to all inguinal nodes with this technique. The result is that a dissection that uses the boundaries of the femoral triangle and is carried out to the level of the cribriform fascia with optimal traction on the lymphovascular fat bundle of the inguinal area will produce a specimen that contains all of the inguinal and femoral nodes.
Frozen-section analysis can be obtained if the initial plan is to perform an ipsilateral LND and suspicious nodes are encountered during the dissection. If metastatic disease is confirmed, a contralateral LND is performed. Closed-suction drains are then placed in the groin dissection, and the skin incision is closed by means of a running delayed absorbable suture. The LND is typically performed first, and attention is then turned to the vulvectomy.
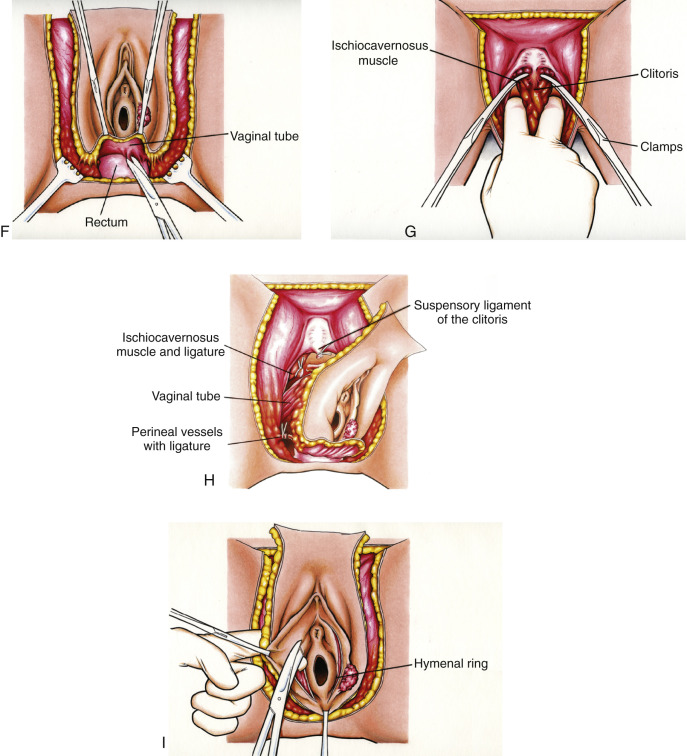
A complete radical vulvectomy is accomplished by defining the margin of dissection. The mons is incised anteriorly, extending medially to the genitocrural fold and posteriorly midway between the anus and posterior fourchette. A bloodless space can be dissected between vulvar fat and the subcutaneous tissue of the thigh using a finger dissection (see Figs. 8.12, C and D ). The tissue is transected and ligated with 0 or 2-0 delayed absorbable suture at the level of the fascia of the thigh (see Fig. 8.12, E ). The posterior dissection is performed sharply (see Fig. 8.12, F ). Special attention is directed to the location of the anus and rectum. It is sometimes helpful for the operator to place a double-gloved finger in the rectum to ascertain its location and avoid damage during this part of the procedure. The clitoris is then isolated, and its suspensory ligament is clamped, divided, and suture ligated at its inferior attachment to the pubic bone. It is often helpful at this point to attempt to isolate the ischiocavernosus muscle and divide this structure as laterally as possible (see Fig. 8.12, G ). The pudendal artery and vein are ligated bilaterally. At this point in the procedure, only the vagina remains attached to the vulva (see Fig. 8.12, H ). A decision about the amount of vagina to be removed should be made relative to the location and size of cancer and based on knowledge of the lymphatic drainage of the vulva. Every effort should be made to avoid resection of the urethra unless it is close to the cancer. If it is indicated, the distal 1 to 2 cm of this organ can be removed without damage to the functional sphincter. The perineal defect is closed primarily with mattress sutures of 0 or 2-0 delayed absorbable suture. Tension on this closure can be prevented, if necessary, by sharp and blunt mobilization of the vaginal barrel or subcutaneous tissue of the thigh. The most anterior extent of the dissection may be allowed to granulate secondarily for large defects if closure would result in significant tension. This prevents distortion of the urethra and alteration of the urinary stream. If the defect is too large for primary closure, intraposition of a split-thickness skin graft or rhomboid or rotational flaps can be used.
Multiple studies have demonstrated that the classical radical vulvectomy can be safely modified depending on the laterality and extent of the lesion without compromising outcomes if a 1 to 2-cm circumferential margin is maintained. If a unilateral lesion is present, radical local excision or modified radical vulvectomy can be performed. If presurgical clinical examination reveals tumor less than 1.0 cm from structures that will not be surgically removed, then the potential of an inadequate surgical margin is high, and preoperative radiation or concomitant chemotherapy and radiation should be considered.
Morbidity Associated With Treatment of Vulvar Carcinoma
In the early series of Way, the operative mortality rate approached 20%. In the past 2 decades, this has been reduced to 1% to 2%. This procedure is commonly carried out in the ninth and tenth decades of life with surprising safety. However, morbidity remains high and most commonly includes wound breakdown, lower extremity lymphedema, lymphocyst formation, decreased pelvic support, and decreased sexual function.
Wound breakdown occurs in more than 50% of patients in most series. This aspect of morbidity is usually limited to skin loss at the margin of the groin incision. Podratz and colleagues at the Mayo Clinic noted impaired primary wound healing in 148 of 175 patients (85%) who were treated with radical vulvectomy and inguinal lymphadenectomy. Removing lesser amounts of skin and decreasing the undermining of the skin flaps have reduced the incidence of wound breakdown. Routine prophylactic antibiotics and the use of closed-suction drainage aid in wound healing and decrease the incidence of wound cellulitis and wound breakdown. Careful débridement and vigorous care to keep the wounds clean and dry optimize healing. Bed rest for 1 to 3 days after extensive resections may be considered but must be balanced against the increased risks of thromboembolism and pulmonary complications.
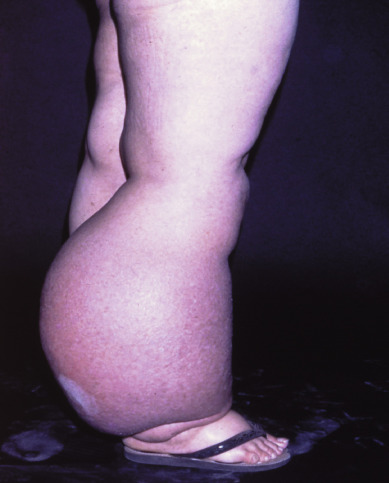
Lymphedema of the lower extremities is another potential major problem (see Fig. 8.17 ). Historically, lymphedema was much more common in patients who underwent inguinal and pelvic lymphadenectomy. Podratz and colleagues reported that varying degrees of lymphedema of the lower extremities occurred in 69% of their patients. This debilitating long-term complication can be reduced by routine use of custom-made elastic support hose during the first postoperative year while collateral pathways of lymph drainage develop. Streptococcal lymphangitis in the lower extremities is associated with lymphedema; thus, Rutledge and colleagues recommended low-dose prophylactic antibiotic therapy after lymphadenectomy. Zhang and colleagues confirmed Plaxe’s observation that sparing of the saphenous vein reduces the incidence of chronic lymphedema and does not compromise the outcome, with similar recurrence rates in patients undergoing complete resection versus preservation of the saphenous vein during inguinal lymphadenectomy.