3 Invasive Breast Cancer
Introduction
This chapter discusses the pathology of invasive breast carcinoma. It is divided into four parts. Part 1 is a discussion of the criteria necessary to make the diagnosis of invasive breast carcinoma. This includes a discussion of the histologic criteria of invasive carcinoma, the mimics of invasive carcinoma, and the various methods used to distinguish invasive carcinoma from its mimics. Part 2 is a description of the items that need to be included in a pathology report of invasive breast carcinoma. Part 3 covers the various histologic types of invasive breast carcinoma and their significance. Part 4 includes a discussion of testing for adjuvant hormone therapy.
Biopsy Procedures
A variety of methods are available for the evaluation of breast lesions. Fine-needle aspirations (FNA) can be performed in the office by either the surgeon or the pathologist, and the only equipment required includes disposable needles, disposable plastic syringes, glass slides, and proper fixative. Proper and rapid fixation is imperative on an FNA specimen, and if the surgeon is to perform the procedure in the office, coordination with and assistance from the laboratory are important. The results can generally be interpreted in a matter of hours. Although FNA cytology has many advantages (it is inexpensive and noninvasive and can be interpreted within a matter of hours), the procedure has several drawbacks. First, the architecture of the lesion is not preserved, and the pathologist cannot make a definitive assessment of the presence or absence of invasion. The assessment of the various types of proliferative lesions such as typical ductal hyperplasia and atypical ductal hyperplasia is also limited in aspirates. In addition, tumors with significant amounts of fibrous tissue frequently provide low cellularity on needle aspirations. The amount of tissue sampled and the pathologist’s experience with aspiration biopsies are also factors to consider. In fact, misinterpretations of breast FNAs have been a leading cause of malpractice lawsuits for pathologists. A National Cancer Institute–sponsored consensus conference on FNA of the breast recommended a so-called triple test approach in which the needle aspiration diagnosis is correlated with the clinical and radiologic findings.1
Needle core biopsy offers greater sampling accuracy than FNA because the architecture of the areas of concern is preserved, and this allows the pathologist to assess invasion. In addition, ancillary studies such as hormone receptor and human epidermal growth hormone receptors (HER2/neu) studies can be performed on material from needle core biopsies, although these studies are probably best performed on larger specimens such as excisional biopsies as the amount of invasive carcinoma may be limited in a core biopsy. While more accurate than FNA, core biopsy also has several limitations:
Although the National Comprehensive Cancer Network (NCCN) guidelines now recommend core biopsy before surgery, in cases with discordant results the definitive method for obtaining tissue is excisional biopsy. Good cooperation between the operating room staff and the histopathology laboratory is required to optimize handling of the surgical specimen. The specimen should be provided to pathology oriented and in the fresh state (Fig. 3-1). On receipt of the specimen, the pathology staff inks the margins for orientation.
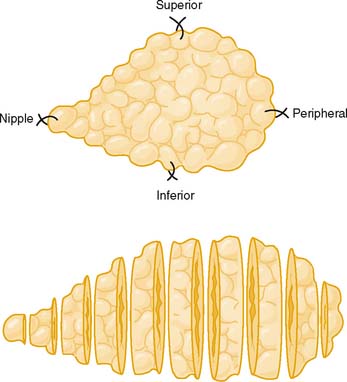
Figure 3-1 Optimal orientation and sectioning of an excisional breast biopsy.
(From Tavassoli FA: Pathology of the Breast, 2nd ed. New York: McGraw-Hill, 1999, Fig. 4-5.)
Breast is one of the most difficult specimens to process in the histopathology laboratory because of its high adipose tissue content. Proper fixation is crucial to optimize the quality of the slides. Ideally, the specimen should be serially sectioned at 2- to 3-mm intervals within several hours of receipt and placed in an adequate amount of fixative. Leaving an unsliced specimen in fixative can lead to poor tissue preservation and ultimately suboptimal histologic sections. It has also been shown that a delay in fixation of as little as 6 hours can result in a decline in the number of mitotic figures and thus lead to inaccuracy in grading.2 Recently, the American Society of Clinical Oncology/College of American Pathologists recommended that for optimal results for HER2/neu testing in breast cancer tissue from excisional biopsies, the specimen should be fixed in neutral buffered formalin for no less than 6 hours and more than 48 hours.3 Needle core biopsies should be fixed for at least one hour.
Histopathologic Appearance of Invasive Breast Cancer
Microscopic Appearance
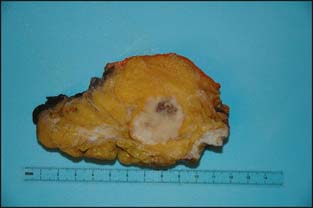
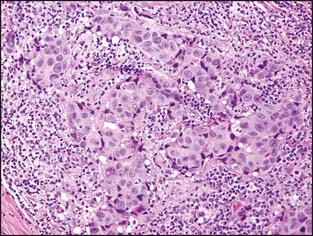
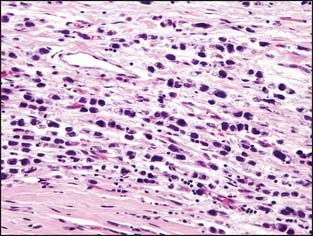
There is considerable variation in the macroscopic appearance of invasive breast carcinoma, based on the histologic type. In the most common type of invasive breast carcinoma— invasive ductal carcinoma not otherwise specified (NOS)—the macroscopic appearance is a firm, white mass that is denser than the surrounding fibroadipose breast tissue. The mass typically has a stellate appearance, and the borders of the nodule are generally somewhat irregular. Frequently, small white-colored spicules and occasionally foci of calcification are identified. Carcinomas frequently have a coarsely granular texture when cut or scraped with the edge of a blade (Fig. 3-2).
Histologic Appearance
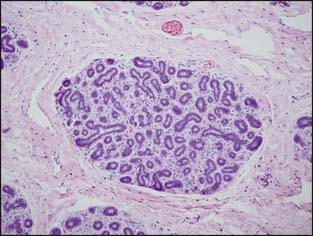
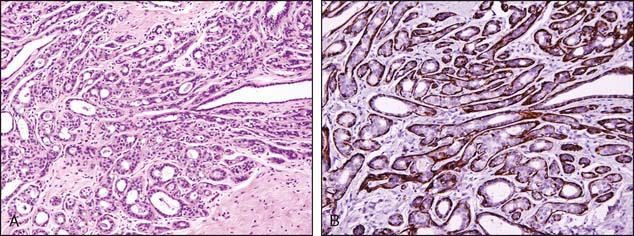
In the normal breast, myoepithelial cells can be easily identified as a flattened layer of cells or as clear cells between the epithelial cells and the stroma (Figs. 3-3 and 3-4A and B). Demonstration of the myoepithelial layer is very helpful for a differentiation of invasive breast cancer from in situ carcinoma. With the exception of the rare entity, microglandular adenosis, the mimickers of invasive carcinoma can usually be separated from invasive breast carcinoma by the presence or absence of the myoepithelial cell layer. Invasive carcinoma also shows breaks in the basement membrane. The presence or absence of the myoepithelial cell layer can usually be determined on hematoxylin and eosin (H&E)–stained sections. However, the myoepithelial cell layer may become obscured as the ductules and acini become distorted by ductal hyperplasia and carcinoma in situ or compressed by stromal sclerosis, thus making identification of the myoepithelial cell layer difficult on H&E stain.
Recently, p63, a homologue of the tumor suppressor protein p53, has gained widespread popularity as a marker for myoepithelial cells. The advantage of using p63 is that it is present only in the nuclei and thus does not show cross-reactivity with myofibroblasts. Two limitations of using p63 have been described in the literature: (1) it occasionally demonstrates an apparently discontinuous myoepithelial layer, particularly around ductal carcinoma in situ (DCIS), and (2) it reacts with a small subset of breast carcinoma tumor cells that show basaloid and squamoid differentiation.4 Figure 3-5A to E illustrates a focus of invasive carcinoma adjacent to noninvasive breast cancer and the use of various immunoperoxidase stains.
The author’s preference is to use a panel of stains that includes p63 and either smooth muscle myosin heavy chain or calponin. Some laboratories use cocktails with dual-staining for smooth muscle myosin heavy chain and p63. Even with most optimal immunoperoxidase stains, there will still be a very small number of cases (around 5%) of DCIS that completely lack myoepithelial cells.5 This underscores the importance of correlating immunohistochemical results with the H&E-stained sections.
Mimickers of Invasive Carcinoma
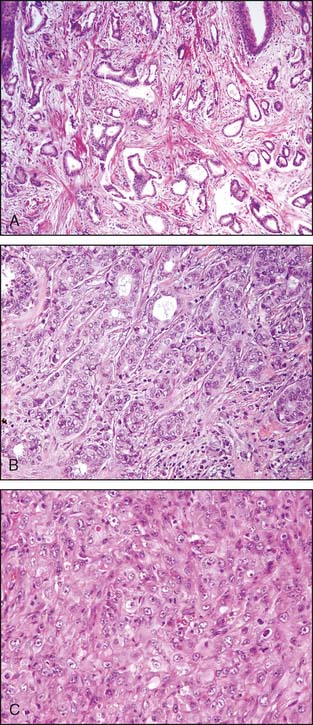
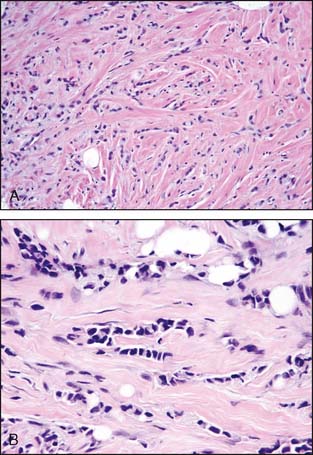
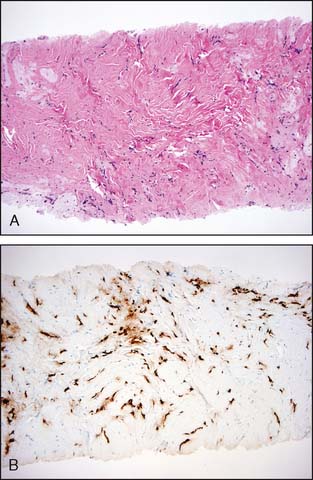
Sclerosing adenosis is the most common form of adenosis and consists of a proliferation of tightly packed ductules. On occasion, sclerosing adenosis may form a mass—a lesion referred to as nodular adenosis. The ductules are closely packed owing to compression of the background collagenous stroma. The compressed and elongated shape of the ductules closely resembles invasive carcinoma. In contrast to the appearance of invasive carcinoma, the ductules maintain a lobulocentric rather than the haphazard distribution that characterizes invasive carcinoma. These features are usually appreciated at low-power microscopic magnification. In addition, a layer of myoepithelial cells and a prominent basement membrane are present between the epithelial cells and the stroma in sclerosing adenosis. The diagnosis can usually be made without the assistance of immunoperoxidase stains, but stains for myoepithelial cells are helpful in difficult cases (Fig. 3-6A and B).
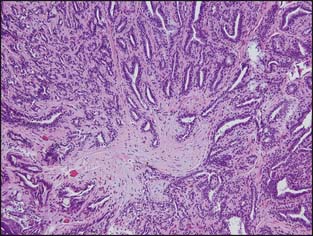
Radial scars (larger forms are sometimes referred to as a complex sclerosing lesion) are closely related to sclerosing adenosis and are composed of entrapped tubules arranged around a central scar (Fig. 3-7). As with sclerosing adenosis, the absence of an invasive pattern and the presence of a layer of myoepithelial cells facilitate the distinction between radial scar and invasive carcinoma.
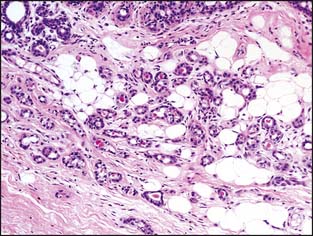
Microglandular adenosis is a rare condition that consists of very compact rounded tubules that are lined by a single layer of cuboidal epithelial cells (Fig. 3-8). The tubules have open lumens and are filled with a colloid-like secretory material. A layer of myoepithelial cells is not present, and the distinction between invasive carcinoma and microglandular adenosis is usually very difficult. The pathologist is able to distinguish microglandular adenosis from invasive tubular carcinoma by the recognition of the characteristic pattern of microglandular adenosis and the absence of a desmoplastic stroma. Fortunately for the pathologist, microglandular adenosis is a rare lesion.
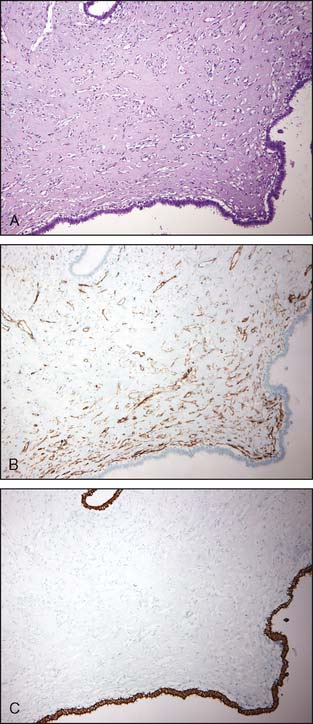
Another benign lesion that can be misinterpreted as invasive carcinoma is pseudoangiomatous stromal hyperplasia (PASH), which consists of interanastomosing, angulated, and slit-like spaces lined by slender spindle cells in a dense collagenous stroma. PASH typically has a distinct H&E morphology; difficult cases may require the use of immunoperoxidase stains (Fig. 3-9A to C). PASH shows immunoreactivity for CD34 but not for cytokeratins. This is illustrated in Figure 3-9B and C.
Occasionally, groups of epithelial cells are mechanically displaced into the surrounding adipose tissue or stroma during a previous biopsy. This is commonly seen after needle core biopsies of papillary lesions6 and can cause difficulty in the assessment of invasion on subsequent excisional biopsy. The absence of a desmoplastic stroma, the absence of adjacent breast structures, the similarity of the cells to a main lesion elsewhere in the excision, and the history are all helpful in distinguishing displaced epithelial cells from invasive carcinoma. Evidence of trauma in the form of blood or hemosiderin immediately adjacent to the suspicious focus is also helpful.
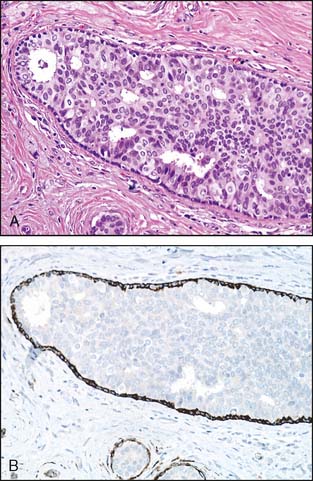
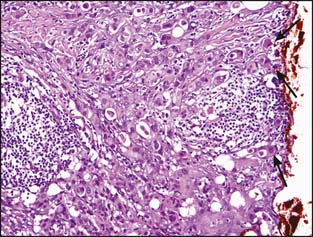
An additional problem has recently been described in large intracystic (in situ) papillary carcinomas. Intracystic papillary carcinomas have traditionally been considered a variant of DCIS. As illustrated in Figure 3-10A and B, the lesions typically have smooth and well-rounded borders and lack the haphazard pattern of invasive carcinoma. However, despite the use of multiple antibodies, a layer of myoepithelial cells between the epithelial cells and the stroma cannot be demonstrated in many instances. Whether or not these lesions are in situ lesions in which the myoepithelial cell layer has become markedly attenuated or represent circumscribed, encapsulated nodules of invasive papillary carcinoma has been the subject of some discussion in the literature.7,8 Currently available outcome data indicate that these lesions have a good prognosis with adequate local therapy alone. One reference recommended that these lesions be referred to as “encapsulated papillary carcinoma” and that they continue to be managed as DCIS until further evidence indicates otherwise.8
PART 2. THE SURGICAL PATHOLOGY REPORT
To standardize reporting of breast carcinoma and ensure that all relevant information for the clinician is included in the pathology report, the College of American Pathologists (CAP) provides a series of surgical pathology cancer protocols and checklists on all organ sites. These are based on the AJCC Cancer Staging Manual, 7th edition.9 CAP protocols are periodically updated and can be accessed via the internet at www.cap.org. An additional article that addresses many of the problematic areas in staging was recently written by Connolly.10
In patients who have undergone multiple core biopsies, the original tumor size should be reconstructed on the basis of a combination of imaging and all histologic findings. Measuring only the residual lesion or the largest size on the core biopsy may result in significant underclassification of the T component.
Grading
An association between tumor grade and survival has been noted since the 1920s and 1930s.11,12 The current system of grading is the modification of the early works of Patey and Scarff13 and Bloom and Richardson14 and most recently the modification by Elston and Ellis.15 In this system, also known as the Nottingham system, the following three parameters are assessed: (1) the degree of tubule formation, (2) the degree of nuclear pleomorphism, and (3) the total number of mitoses per 10 high-power fields (hpf). A score between 1 and 3 is assigned to each of the parameters. The three scores are added together, and a grade is assigned on the basis of the total number. Tumors that show greater than 75% tubule formation are assigned a score of 1; tumors that show between 10% and 75% tubule formation are assigned a score of 2; and tumors that show less than 10% tubule formation are assigned a score of 3.
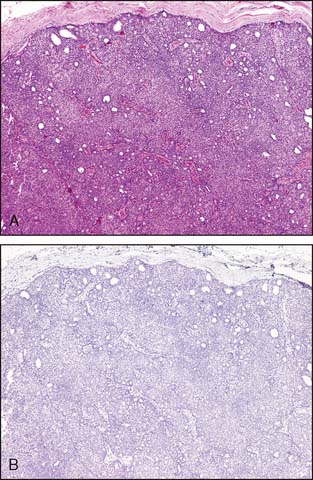
The nuclear grade is probably the most subjective aspect of the scoring. Nuclei that are of uniform size and shape are scored as 1. Bizarre nuclei showing marked variation are scored as 3, and a score of 2 is given when there are intermediate variations in nuclear characteristics. Mitotic counts are evaluated as the number of mitotic figures found in 10 consecutive hpf in the most mitotically active part of the tumor. Field selection for mitotic counting should be from the peripheral leading edge of the tumor. Only clearly identifiable mitotic figures should be counted; hyperchromatic, karyorrhectic, or apoptotic nuclei are excluded. Since the area of the microscopic field may vary according to the microscope used, mitotic counts require standardization by converting the number of mitoses per 10 hpf into the number of mitoses per set area (such as a square millimeter), as described by Kuopio and Collan16 or by using a grid system (Fig. 3-11). Examples of the various grades of invasive carcinoma are illustrated in Figure 3-12A to C.
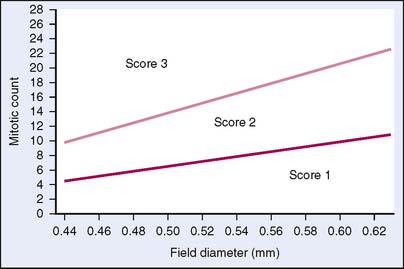
Figure 3-11 Graph of number of mitotic figures per 10 hpf by field diameter.
(Reproduced with permission of authors and publisher from NHSBSP Publication, NHS Breast Screening Programme, 1997.)
Margin Status
Pathologic evaluation of the margin status is accomplished by the use of inked margins. In most instances, particularly with the advent of breast-conserving surgery, the surgeon identifies the various margins through the use of sutures or clips. This should be documented in the surgical report. The pathologist then uses different colors of ink to label the six margins and section the specimen along the longest axis of the specimen. In some instances, the surgeon does not provide any sutures or clips to orient the specimen. The pathologist then inks the entire outer surface of the specimen in one color. A positive margin can be identified when there is tumor against the ink (Fig. 3-13). It is also recommended that the pathologist provide the closest distance of the tumor to the inked margins on each of the six sides of the specimen.
The use of ink to identify margins presents several technical challenges. First, the surface of a breast surgical specimen is generally not flat and smooth but frequently irregular with numerous small crevices that ink may run into. In addition, ink frequently runs and can mark tissue edges for which it was not intended. The use of various mordants such as Bouin’s solution helps to bind the inks to the tissue surfaces. Ink seeping into crevices or running off the surface can result in a falsely reported positive margin. The recommended distance between the tumor and the inked surface to classify it as a negative margin varies from no tumor at the inked surface up to 1 cm. There is no national consensus on the acceptable distance for classification of a negative margin. However, all groups agree that margins should be assessed and that there should be no tumor at the inked surface as a minimum standard.
Invasive Carcinoma with an Extensive in Situ Component
CAP recommends that for breast carcinomas that have both an invasive and an in situ component, the pathology report should specify whether an extensive in situ (intraductal) component (EIC) is present. EIC is present when DCIS comprises more than 25% of the main tumor and extends beyond the boundaries of the invasive tumor and into the surrounding breast parenchyma. This finding is associated with an increased risk of local recurrence when the surgical margins are positive.17 EIC appears to have less significance when DCIS does not extend close to any of the margins after careful histologic evaluation.18 The significance of this finding relates to the fact that in situ lesions, unlike most invasive lesions, frequently do not form a grossly apparent fibrous mass. Thus, the notation “EIC” serves to inform the treating physician that in situ carcinoma may extend beyond the boundaries of the grossly apparent tumor mass and may require more extensive excision. Correlating mammograms with the pathologic findings and assessing surgical margins are particularly important steps when treating patients with EIC.
Lymphovascular Space Invasion
Peritumoral vascular invasion should be noted because this condition has been associated with local failure and reduced overall survival (Fig. 3-14). Distinguishing between lymphatic channels and blood vessels is not necessary. However, it is important for lymphovascular space invasion to be distinguished from tissue retraction, as is commonly seen in invasive micropapillary carcinoma. The use of various stains for endothelial cells such as CD31 can be of assistance.
Lymph Nodes
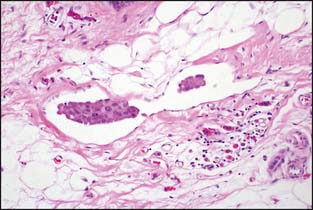
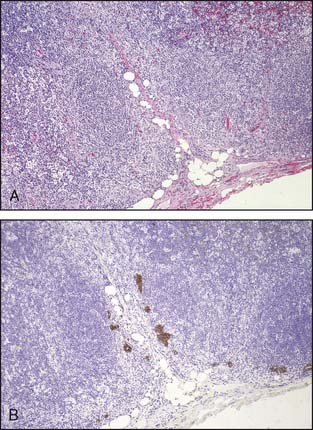
Lymph node metastases consist of collections of tumor cells in either solid or tubular arrangement within the lymph node tissue (Fig. 3-15). These are mostly identified just beneath the lymph node capsule and are generally easily identified on H&E sections. The pathology report should clearly state the total number of lymph nodes examined, the total number of nodes involved, and the greatest dimension of the largest metastatic focus. Grossly uninvolved nodes should be submitted in their entirety for histologic evaluation. Representative sections of grossly positive nodes may be submitted, with a single microscopic section from each lymph node considered sufficient. Extranodal tumor extension, if present, should be included in the pathology report.
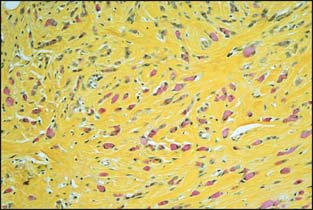
Recently, there has been considerable discussion on the significance of very minute metastatic foci and the use of immunohistochemistry to identify metastatic foci. As illustrated in Figure 3-16A and B, cytokeratin stains can greatly assist in the identification of lymph node metastasis. This is particularly true for invasive lobular carcinoma, in which the metastatic foci can be very subtle. However, the significance of these microscopic foci, particularly those identified only with the assistance of immunohistochemical stains, has been the subject of considerable debate.
The current AJCC staging manual defines isolated tumor cells (ITCs) as single cells or small clusters of cells not larger than 0.2 mm, usually with no histologic evidence of malignant activity such as a stromal reaction9 (Fig. 3-17). Because isolated tumor cells are sometimes dispersed throughout the entire node rather than in a single focus, making a measurement virtually impossible, the recent AJCC manual has expanded the definition of ITCs to also include cases in which there are fewer than 200 individual tumor cells in a single histologic section of a lymph node.9 If morphologic techniques (including immunohistochemistry) are used to detect isolated tumor cells, the regional lymph nodes should be designated as pN0(i+) or pN0(i-), depending on whether the immunohistochemical stains are positive. If nonmorphologic (molecular) methods are used, the nodes are designated as pN0(mol-) or pN0(mol+) as appropriate.
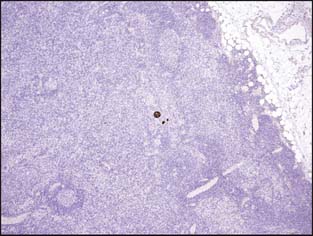
Figure 3-17 Isolated tumor cells in a lymph node identified by cytokeratin staining (brown color) pN0(i+).
PART 3. HISTOLOGIC TYPES OF INVASIVE BREAST CARCINOMA
Invasive carcinomas are divided into two large categories based on the histology: invasive ductal and invasive lobular. Many of the histologic subtypes, such as tubular carcinoma, mucinous (colloid) carcinoma, medullary carcinoma, invasive papillary carcinoma, invasive micropapillary carcinoma, and invasive apocrine carcinoma, are considered special variants of invasive ductal carcinoma. As with in situ lesions, the designations invasive ductal and invasive lobular refer to a growth pattern and do not necessarily imply a site of origin. A morphologic study by Wellings and colleagues19 demonstrated that most breast carcinomas—both ductal and lobular—originate in the terminal duct lobular unit.
Invasive Ductal Carcinoma
The current World Health Organization (WHO) classification requires that for a tumor to be typed as invasive ductal carcinoma, NOS, it must have a nonspecialized pattern in over 50% of its mass. If the ductal NOS component makes up between 10% and 49% of the tumor, with the rest of the tumor composed of one recognized histologic variant, then the tumor is to be classified as mixed ductal carcinoma and special type.20
Invasive Lobular Carcinoma
WHO defines invasive lobular carcinoma as an invasive carcinoma usually associated with lobular carcinoma in situ and composed of noncohesive cells individually dispersed or arranged in single-file linear pattern in a fibrous stroma. Pure invasive lobular carcinomas represent about 4% to 5% of all invasive breast carcinomas.20 There has been an increase in the incidence of invasive lobular carcinoma in women over 50,21 and a causal relationship with hormone replacement therapy has been suggested.22–24
The classic pattern of invasive lobular carcinoma is illustrated in Figure 3-18A and B. The most recognizable feature is the single-file pattern of invasion. The cells are generally small, with a mild to moderate degree of nuclear pleomorphism (grade 1 or 2). The nucleus is round and regular with small to inconspicuous nucleoli. Intracytoplasmic lumens are present in some of the cells with intracytoplasmic mucin, which is highlighted by special stains (Fig. 3-19). Invasive lobular carcinoma is frequently encountered in biopsies with lobular intraepithelial neoplasia (LIN), particularly the higher-grade LIN lesions.25
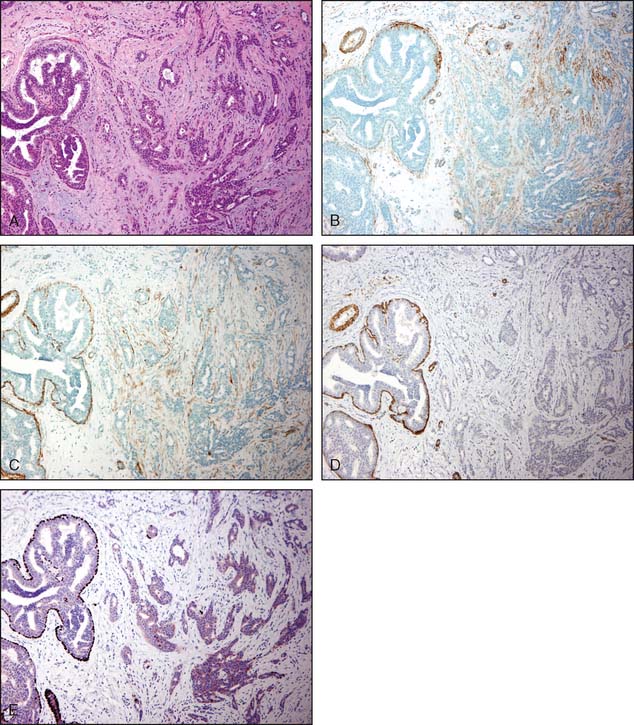
The pathologist must be very careful not to overlook small foci of invasive lobular carcinoma, because the desmoplastic stromal response and lymphocytic response that frequently accompany other forms of invasive carcinoma are often not present. In about 25% of all cases of invasive lobular carcinoma, a palpable mass or mammographic abnormality may not be detected. In addition, the small size and shape of the cells in many cases of invasive lobular carcinoma may resemble lymphocytes. Immunoperoxidase stains for cytokeratins may be helpful in selected cases. Figure 3-20A and B illustrates a very difficult and subtle case of invasive lobular carcinoma in which immunoperoxidase staining for cytokeratin facilitates the diagnosis.
Unlike invasive ductal carcinomas, the cells of classic invasive lobular carcinomas lack cell-to-cell cohesion and are described as having a discohesive or loosely cohesive pattern of growth. This is related to the lack of expression of the transmembrane adhesion molecule Ecadherin. Recent genetic data have noted deletions at chromosomal region 16q22 in a high percentage of invasive lobular carcinoma. This genetic locus harbors the E-cadherin molecule.26 Whether E-cadherin staining should be used in distinguishing invasive lobular from invasive ductal carcinomas is the subject of considerable debate, particularly in cases that show the classic pattern of invasive lobular carcinoma. In a small percentage of tumors, it will be impossible to make a definitive distinction between invasive ductal or invasive lobular carcinoma.
In the pleomorphic pattern of invasive lobular carcinoma, the cells maintain their single-file pattern of invasion and lack of cohesion, but show significantly more pleomorphism than in the classic variant (Fig. 3-21). Pleomorphic invasive lobular carcinoma tends to occur in older women and is generally associated with a more aggressive clinical course than the classic pattern of invasive lobular carcinoma.27–29 One study noted a greater incidence of expression with markers for aggressive behavior such as p53 and HER2 in the pleomorphic pattern of invasive lobular carcinoma than in the classic variant.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree