The concept of immunosurveillance of cancer has been widely accepted for many years, but only recently have the precise mechanisms of tumor-host immune interactions been revealed. Inflammatory and immune reactions play a role in melanomagenesis, and may contribute to the eradication of tumor as well as potentiating its growth and proliferation. Studies of the role of tumor–immune system interactions are providing insights into the pathogenesis and opportunities for highly effective therapeutic strategies. Some patients, even with advanced disease, are now cured with immunotherapy, and increasing numbers of such cures are likely in future.
Key points
- •
Inflammation and immune reactions, particularly involving the innate immune system, are important elements in melanomagenesis.
- •
Host and tumor characteristics of an immunologic nature determine the fate of melanoma, from the oncogenesis of the primary tumor to the characteristics of the draining lymph node to the microenvironment of melanoma in sites of metastasis.
- •
Therapeutic strategies need to exploit the existing features of the host-tumor immunologic interactions as well as alter selected feature of the tumor and/or immune system to improve treatment outcomes.
- •
The amount of heterogeneity among tumors and the host’s immune reactions to them provide the basis for recognizing that all forms of therapy require careful patient selection to provide the best therapeutic index at all stages of malignancy.
Background
Inflammation and Immunosurveillance in Melanomagenesis
Common knowledge holds that cutaneous melanoma is caused by harmful ultraviolet (UV) radiation, largely from intermittent scorching sunburns, particularly in childhood, an observation based on epidemiologic associations. The contribution of intentional exposure to erythema-inducing doses of UV wavelengths from tanning beds has been the focus of efforts to alter sun behaviors and enact legislation against access, particularly by minors, to tanning salons. It is also commonly thought that cancer immunosurveillance plays a large role in preventing or reducing the risk of invasive cancers, and there are plentiful examples in the literature for a strong association between immunosuppression or immunodeficiency and the occurrence of malignancy. However, despite a large and growing role for immunomodulatory therapies in advanced stages, melanoma is not one of the neoplasms occurring at significantly increased frequency among patients with compromised immunity. It is likely that the precise type and severity of immunodeficiency required to promote melanomagenesis or emergence of invasive, overt disease from a precursor or indolent disease occur only rarely.
Inflammation plays an important role in melanomagenesis, and inflammation is also a critical component of the liaisons between innate and adaptive immune responses, both of which have been shown to play roles in controlling tumors and protecting against new malignancy. Starting with the earliest stages of melanomagenesis, inflammation induced by UV light is associated with enhanced blood flow, vascular permeability, and damage to subcellular structures resulting from reactive oxygen species. In the earliest example of a tumor microenvironment reactive to oncogenic environmental factors, both the melanocytes and keratinocytes are induced by UV light to produce inflammatory substances that cooperate to prepare for tumor promotion and an immunosuppressive milieu and eventually the elaboration of growth factors that further support tumor growth, invasion, and metastasis.
Immunosuppression induced by UV light contributes to melanomagenesis via the reduction in the number of Langerhans cells; decreased antigen presentation; and elaboration of type 2 cytokines and other substances with suppressive effects, such as interleukin (IL)-4, IL-10, and prostaglandin-E2. UV light also stimulates the production of growth factors with tumor-promoting effects such as alpha-melanocyte–stimulating factor and platelet-activating factor. Neuropilin-1, a member of the vascular endothelial growth factor (VEGF) receptor family, contributes to the protumoral effects of a subset of regulatory T cells in melanoma, and its effects seem to be mediated by transforming growth factor beta (TGF-β) and to be synergistic with those of VEGF.
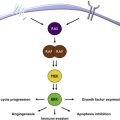
The role of chronic inflammation in melanomagenesis is less clear, although it has been suggested that solar elastosis, a consequence of prolonged rather than acute UV skin damage, may be protective and confer a more favorable prognosis in melanoma ; however, this observation may also be explained by some other favorable feature(s) of melanoma arising as a result of chronic UV damage rather than as a direct result of the solar elastosis. A promising therapeutic target is related to the phenomenon of T-cell immune exhaustion, caused in part by the interactions between ligands on the tumor cell as well as on other inflammatory cells, the programmed-death (PD)-1/PD-ligand (PD-L1) interaction, which is detailed later in this article and elsewhere in this issue by Naidoo and colleagues. Established melanoma is likely to represent successful tumor evolution through the 3 stages of immunoediting described by Schreiber and colleagues, beginning with elimination, evolving to equilibrium, and eventually resulting in escape. Also of likely immunotherapeutic importance are recent observations that the common molecular driver of melanoma and some other tumors, BRAF v600 E , confers alterations in melanocytes resulting in an immunosuppressive microenvironment that can be overcome by inhibitors of the enhanced mitogen-associated protein kinase (MAPK) pathway signaling ( Fig. 1 ). Whether other, less commonly occurring oncogenic drivers are similarly immunosuppressive remains to be shown, and the growing number of molecularly targeted inhibitors need to be carefully tested for their impact on cells of the immune system (both in the circulation and in the tumor microenvironment), because their on-target and off-target effects may be varied and unpredictable. Because molecularly and immunologically targeted therapies and the opportunities for combinatorial strategies are increasing rapidly, it is necessary to examine each component carefully in order to design regimens with the optimal therapeutic index.
Prognostic Value of Studying the Immune Tumor Microenvironment
The importance of immune-mediated events in the response of malignancy to traditional cytotoxic therapies, including chemotherapies and radiation, has recently undergone a resurgence of interest, with evidence that most antitumor therapies work at least in part through immune control and do not work in the absence of immune effectors. Examples of immunogenic chemotherapy have recently been studied by investigators at the Institut Gustav-Roussy (IGR), who analyzed the immunomodulatory effects of many different cytotoxic and targeted anticancer agents. Because there were substantial differences in the effects and mechanisms of different agents on responses, which included both innate and adaptive immune systems, it is not possible to distinguish those drugs that work predominantly through a contribution to immunogenic cancer cell death. Furthermore, the importance of schedule and cell targets (immune effectors, tumor cells, vascular endothelium, and stromal cells) responding to various anticancer drugs needs to be taken into account in designing regimens to exploit these findings. The transcription factor signal transducer of activated T cells-3 (STAT3) may be of particular importance in mediating the expression of a broad spectrum of immunosuppressive, inflammatory, and proangiogenic factors that contribute to the growth and survival of tumor cells and the immunosuppressive state of the peritumoral milieu, and efforts to suppress its activity in combinatorial antitumor strategies have been encouraging.
A series of additional studies from the IGR have provided the foundations for a systematic approach to the definition of immune system–tumor interactions with important implications for prognostic and predictive considerations in the further design of strategies to answer biological questions and to optimize clinical trials. These principles are best illustrated by Fig. 2 . This model describes the immunoscore, a method for quantitating the CD8 effector and memory cells in different locations of the immune tumor microenvironment, which has a strong independent prognostic value (and allows a dichotomization of relapse-free and overall survival) in colorectal cancer and seems to be superior to earlier systems such as the tumor-node-metastasis (TNM) staging systems commonly used for solid tumor prognostication. The immunoscore was derived from a more complex system, termed the immune contexture, including the same T-cell subsets as the immunoscore but also taking into account the orientation, density, organizational characteristics, and functional characteristics of T cells in the tumor microenvironment. The relevance of this approach to the melanoma immune tumor microenvironment and the importance of other nontumor cells such as stromal, vascular, and other cells of immune and inflammatory lineages remains to be elucidated and is also likely to differ depending on the primary location/biology, sites of metastatic disease, driver mutations, and immunogenetics of the patient ( Fig. 3 ).