The concept of immunosurveillance of cancer has been widely accepted for many years, but only recently have the precise mechanisms of tumor-host immune interactions been revealed. Inflammatory and immune reactions play a role in melanomagenesis, and may contribute to the eradication of tumor as well as potentiating its growth and proliferation. Studies of the role of tumor–immune system interactions are providing insights into the pathogenesis and opportunities for highly effective therapeutic strategies. Some patients, even with advanced disease, are now cured with immunotherapy, and increasing numbers of such cures are likely in future.
Key points
- •
Inflammation and immune reactions, particularly involving the innate immune system, are important elements in melanomagenesis.
- •
Host and tumor characteristics of an immunologic nature determine the fate of melanoma, from the oncogenesis of the primary tumor to the characteristics of the draining lymph node to the microenvironment of melanoma in sites of metastasis.
- •
Therapeutic strategies need to exploit the existing features of the host-tumor immunologic interactions as well as alter selected feature of the tumor and/or immune system to improve treatment outcomes.
- •
The amount of heterogeneity among tumors and the host’s immune reactions to them provide the basis for recognizing that all forms of therapy require careful patient selection to provide the best therapeutic index at all stages of malignancy.
Background
Inflammation and Immunosurveillance in Melanomagenesis
Common knowledge holds that cutaneous melanoma is caused by harmful ultraviolet (UV) radiation, largely from intermittent scorching sunburns, particularly in childhood, an observation based on epidemiologic associations. The contribution of intentional exposure to erythema-inducing doses of UV wavelengths from tanning beds has been the focus of efforts to alter sun behaviors and enact legislation against access, particularly by minors, to tanning salons. It is also commonly thought that cancer immunosurveillance plays a large role in preventing or reducing the risk of invasive cancers, and there are plentiful examples in the literature for a strong association between immunosuppression or immunodeficiency and the occurrence of malignancy. However, despite a large and growing role for immunomodulatory therapies in advanced stages, melanoma is not one of the neoplasms occurring at significantly increased frequency among patients with compromised immunity. It is likely that the precise type and severity of immunodeficiency required to promote melanomagenesis or emergence of invasive, overt disease from a precursor or indolent disease occur only rarely.
Inflammation plays an important role in melanomagenesis, and inflammation is also a critical component of the liaisons between innate and adaptive immune responses, both of which have been shown to play roles in controlling tumors and protecting against new malignancy. Starting with the earliest stages of melanomagenesis, inflammation induced by UV light is associated with enhanced blood flow, vascular permeability, and damage to subcellular structures resulting from reactive oxygen species. In the earliest example of a tumor microenvironment reactive to oncogenic environmental factors, both the melanocytes and keratinocytes are induced by UV light to produce inflammatory substances that cooperate to prepare for tumor promotion and an immunosuppressive milieu and eventually the elaboration of growth factors that further support tumor growth, invasion, and metastasis.
Immunosuppression induced by UV light contributes to melanomagenesis via the reduction in the number of Langerhans cells; decreased antigen presentation; and elaboration of type 2 cytokines and other substances with suppressive effects, such as interleukin (IL)-4, IL-10, and prostaglandin-E2. UV light also stimulates the production of growth factors with tumor-promoting effects such as alpha-melanocyte–stimulating factor and platelet-activating factor. Neuropilin-1, a member of the vascular endothelial growth factor (VEGF) receptor family, contributes to the protumoral effects of a subset of regulatory T cells in melanoma, and its effects seem to be mediated by transforming growth factor beta (TGF-β) and to be synergistic with those of VEGF.
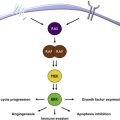
The role of chronic inflammation in melanomagenesis is less clear, although it has been suggested that solar elastosis, a consequence of prolonged rather than acute UV skin damage, may be protective and confer a more favorable prognosis in melanoma ; however, this observation may also be explained by some other favorable feature(s) of melanoma arising as a result of chronic UV damage rather than as a direct result of the solar elastosis. A promising therapeutic target is related to the phenomenon of T-cell immune exhaustion, caused in part by the interactions between ligands on the tumor cell as well as on other inflammatory cells, the programmed-death (PD)-1/PD-ligand (PD-L1) interaction, which is detailed later in this article and elsewhere in this issue by Naidoo and colleagues. Established melanoma is likely to represent successful tumor evolution through the 3 stages of immunoediting described by Schreiber and colleagues, beginning with elimination, evolving to equilibrium, and eventually resulting in escape. Also of likely immunotherapeutic importance are recent observations that the common molecular driver of melanoma and some other tumors, BRAF v600 E , confers alterations in melanocytes resulting in an immunosuppressive microenvironment that can be overcome by inhibitors of the enhanced mitogen-associated protein kinase (MAPK) pathway signaling ( Fig. 1 ). Whether other, less commonly occurring oncogenic drivers are similarly immunosuppressive remains to be shown, and the growing number of molecularly targeted inhibitors need to be carefully tested for their impact on cells of the immune system (both in the circulation and in the tumor microenvironment), because their on-target and off-target effects may be varied and unpredictable. Because molecularly and immunologically targeted therapies and the opportunities for combinatorial strategies are increasing rapidly, it is necessary to examine each component carefully in order to design regimens with the optimal therapeutic index.
Prognostic Value of Studying the Immune Tumor Microenvironment
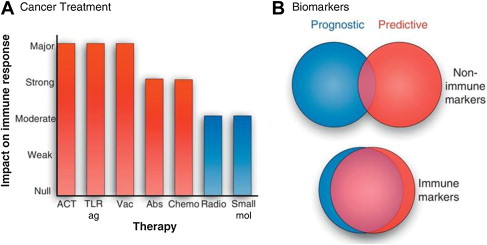
The importance of immune-mediated events in the response of malignancy to traditional cytotoxic therapies, including chemotherapies and radiation, has recently undergone a resurgence of interest, with evidence that most antitumor therapies work at least in part through immune control and do not work in the absence of immune effectors. Examples of immunogenic chemotherapy have recently been studied by investigators at the Institut Gustav-Roussy (IGR), who analyzed the immunomodulatory effects of many different cytotoxic and targeted anticancer agents. Because there were substantial differences in the effects and mechanisms of different agents on responses, which included both innate and adaptive immune systems, it is not possible to distinguish those drugs that work predominantly through a contribution to immunogenic cancer cell death. Furthermore, the importance of schedule and cell targets (immune effectors, tumor cells, vascular endothelium, and stromal cells) responding to various anticancer drugs needs to be taken into account in designing regimens to exploit these findings. The transcription factor signal transducer of activated T cells-3 (STAT3) may be of particular importance in mediating the expression of a broad spectrum of immunosuppressive, inflammatory, and proangiogenic factors that contribute to the growth and survival of tumor cells and the immunosuppressive state of the peritumoral milieu, and efforts to suppress its activity in combinatorial antitumor strategies have been encouraging.
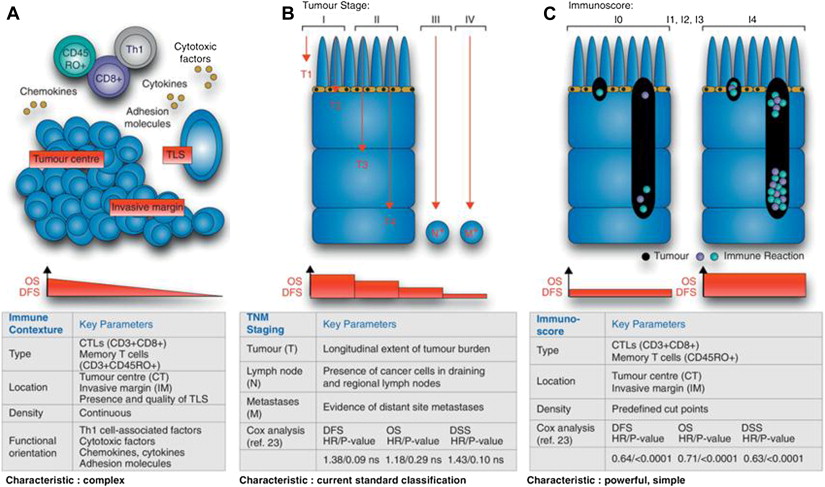
A series of additional studies from the IGR have provided the foundations for a systematic approach to the definition of immune system–tumor interactions with important implications for prognostic and predictive considerations in the further design of strategies to answer biological questions and to optimize clinical trials. These principles are best illustrated by Fig. 2 . This model describes the immunoscore, a method for quantitating the CD8 effector and memory cells in different locations of the immune tumor microenvironment, which has a strong independent prognostic value (and allows a dichotomization of relapse-free and overall survival) in colorectal cancer and seems to be superior to earlier systems such as the tumor-node-metastasis (TNM) staging systems commonly used for solid tumor prognostication. The immunoscore was derived from a more complex system, termed the immune contexture, including the same T-cell subsets as the immunoscore but also taking into account the orientation, density, organizational characteristics, and functional characteristics of T cells in the tumor microenvironment. The relevance of this approach to the melanoma immune tumor microenvironment and the importance of other nontumor cells such as stromal, vascular, and other cells of immune and inflammatory lineages remains to be elucidated and is also likely to differ depending on the primary location/biology, sites of metastatic disease, driver mutations, and immunogenetics of the patient ( Fig. 3 ).
Inflammatory and Immune Gene Signatures in the Biology of Melanoma
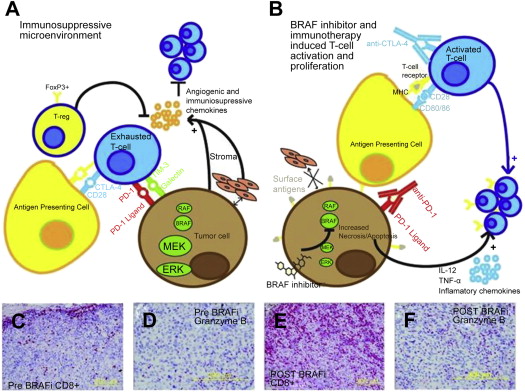
Extensive studies of melanoma gene expression have supported the concept that it may be possible to recognize a dichotomy of biological behaviors characterized by patterns of gene expression reflecting differences in the immunologic interactions among tumor cells, stroma, and infiltrating immune and inflammatory cells. In an attempt to elucidate why some melanoma metastases seem to be heavily infiltrated by CD8 (potentially cytotoxic) T cells but nevertheless grow and eventually kill the patient, whereas other lethal melanomas do not feature an immune cell infiltrate, investigators at the University of Chicago described 2 distinct patterns of gene expression, one termed inflamed gene signature and the other noninflamed. In addition to elegantly working out the cellular and molecular biology of the associated cell-cell interactions through gamma-interferon, intracellular sensing of tumor DNA, and chemokines that mediate T-cell homing to tumor, these investigators proposed that the biology underlying melanoma growth despite an inflamed gene signature depends on the emergence of an exhaustion phenotype (thus identifying an immunologic target for which therapy is already available [antibodies to PD-1 or PD-L1]) or other suppressive mediators such as indole dioxygenase, for which therapeutic small molecule inhibitors are already undergoing clinical testing ( Fig. 4 ).
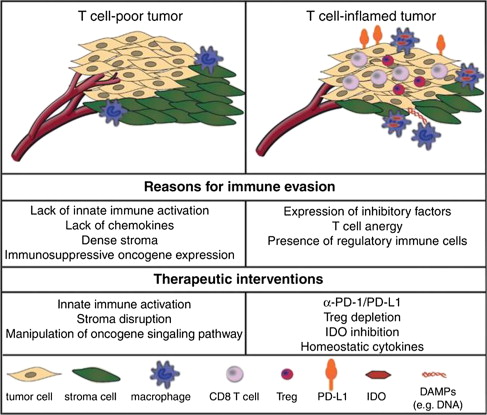
Additional forms of T-cell anergy, tolerance, or immunologic ignorance of tumor also exist (detailed later in this article) and may be possible to overcome therapeutically using other immune checkpoint blockers, such as anti–cytotoxic T lymphocyte antigen-4 (CTLA-4), immunostimulatory/homeostatic cytokines like IL-15, or agonistic antibodies mimicking the action of the natural ligands for costimulatory receptors (OX40, CD137, and CD40L). Pleiotropic factors like TGF-β, IL-10, and the transcriptional regulator STAT3 and its downstream targets are also likely to play important supporting roles in cancer immunotherapy strategies, but their functions and targeting are currently too complex to incorporate into therapeutic strategies. Stimulation of antigen presentation and other important functions mediating crosstalk between cells traditionally part of the innate immune system and those considered responsible for adaptive responses include a wide variety of dendritic cell (DC) signaling agonists derived from pathogen-associated molecular pattern molecules like bacterial cell wall components or nucleic acid fragments, or damage-associated molecular pattern molecules like double-stranded RNA that bind to specialized receptors on or within DCs. Other agents, including the interferons (IFN), both type I (IFN-α and IFN-β) and type II or immune (IFN-γ), can enhance major histocompatibility complex (MHC) (human leukocyte antigen [HLA]) molecule expression on tumor cells and increase the expression and presentation by class I and II MHC molecules of immunogenic peptide epitopes derived from tumor antigens, in part by altering the ratio of different types of proteasomes responsible for antigen processing. IFN-α also enhances the responsiveness of cytotoxic CD8 T cells to homeostatic cytokines such as IL-15 and IL-7, as well as to recall antigens. Many elements of the immune tumor microenvironment influence the processing and transport of antigens within the tumor cell to their final site of presentation in the epitope-binding site of the HLA molecule to the peptide-recognition portion of the T-cell receptor. The expression of some of the genes involved in these tightly regulated pathways, as well as the intracellular transport of tumor antigen–derived peptides, can be modulated therapeutically by drugs that are already in the clinic or in development. These drugs include demethylating agents like 5-aza-2-deoxycytidine and investigational heat-shock protein inhibitors that can alter intracellular patterns of antigen transport.
Role of the Immune System in Dissemination of Melanoma to the Nodes and Distant Organs
Among the critical unresolved questions regarding the biology of melanoma is the control of dissemination to regional lymph nodes and the role played by cells and molecules of the immune system. It is increasingly evident that lymph nodes are not merely passive recipients of melanoma cells as they traverse the local lymphatics but that important intercellular interactions and intracellular events also control this phenomenon, which is likely set in motion before the appearance of melanoma cells in the node. Homing to the nodes is controlled in part by the expression of interacting pairs of chemokines and their receptors, and other intercellular interactions are mediated by adhesion molecules that allow melanoma cells to interact with endothelial cells. Important interactions with the vasculature are also mediated by inflammatory cytokines, VEGFs, and other vasoactive molecules. Lymph nodes may play a more active role in the dissemination of melanoma cells, via the elaboration of chemokine 1 (CCL1) by the lymphatic endothelial cells, which binds to chemokine receptor 8 (CCR8) on tumor cells, inducing chemotaxis to the node and controlling the transmigration of tumor cells from the subcapsular sinus to the cortex of the node.
Recent studies have shown that premetastatic lymph nodes from patients with melanoma have alterations in their T-cell receptor function as shown by the expression of the zeta subunit of the CD3 signaling molecule. Established nodal metastases also feature cellular infiltrates characteristic of chronic inflammation, including regulatory T cells (T reg ), myeloid-derived suppressor cells, tolerogenic DCs, and tumor-associated macrophages (TAM), which produce suppressive small molecules and other factors that mediate immunosuppression. Further studies of melanoma-positive sentinel lymph nodes revealed that those from patients with either additional positive nodes at completion of lymph node dissection or from patients who relapse within 5 years differed significantly in their numbers of CD30+ lymphocytes from those who experienced neither unfavorable outcome. The CD30+ cell population consisted of a heterogeneous distribution of cells with phenotypic markers of suppression or exhaustion and that were also found in the peripheral blood of patients with advanced melanoma. A potential role for activated natural killer (NK) cells in nodal immunosurveillance and adjuvant therapy is supported by the recent report of a unique population of NK cells in melanoma-containing lymph nodes that can be stimulated to high lytic activity by IL-12 or IL-15. The immunophenotypic and functional characteristics of melanoma-draining lymph node cells provides a rich foundation for both prognostic and predictive factors in the design of adjuvant trials for resected melanoma that complements the information increasingly available from the molecular and immunologic features of the primary tumor.
Immunosurveillance Against Primary and Relapsing Melanoma
It has long been thought that some form of immunosurveillance provides protection from cancer and that among the reasons for most cancers’ predilection for older individuals is the gradual erosion of immune competence with advancing age. Recent observations on the impact of known molecular drivers and other modulators of melanoma biology have provided some understanding of different categories of melanoma, a disease that also has a lower median age and affects even children, adolescents, and young adults. It is likely that the role of immunosurveillance and the potential for immunotherapy strategies differ substantially among these different classes of melanoma that take into account not only patient age but also other tumor-host interactions and tumor-intrinsic factors such as their molecular drivers, which also change over time (melanoma has a high rate of acquisition of new mutations). Thus, despite the recent successes resulting from new immunotherapies for advanced melanoma (in particular, immune checkpoint blockade with antibodies as well as therapeutic infusions of expanded autologous cytotoxic T lymphocytes), the control of melanoma at every point along the continuum from melanomagenesis to proliferation and metastasis of advanced tumors requires specific alignment of cellular and molecular determinants of the interactions among these cells (including stroma and vasculature, which are also affected by the complex immunogenetic background of human patients). Thus, although the principles of immunosurveillance and immunotherapy for melanoma share many elements with other tumor types, there remain important differences that make it necessary to design investigational strategies based on the precise requirements for melanoma, and even for different types of melanoma (eg, uveal vs mucosal vs cutaneous). However, it may be possible to find methods to render melanomas more immunogenic using some of the interventions discussed in this article (eg, epigenetic modification of tumor, and radiotherapy and intralesional injection of immunomodulatory molecules) and thus cause it to behave more like those tumors that are known to depend more heavily on the immunosuppressed host, such as Merkel cell carcinoma, or that are virally induced, such as cervical cancer or cancers resulting from both viral and chronic inflammatory contributions (hepatitis B-associated and C-associated hepatocellular cancer).
Despite the presence of identified driver oncogenic mutations in at least 70% of cutaneous melanomas (and likely a high percentage of the others, yet to be identified), these mutations alone are not sufficient to confer the fully malignant phenotype, suggesting that additional modifying factors are required ; however, the role and the precise parameters of the host immune response in preventing or modifying melanomagenesis and subsequent melanoma behavior remains ill defined. To date, there is little evidence that the incidence of melanoma or the likelihood of an unfavorable outcome (earlier relapse, shorter survival) of previously diagnosed melanoma is substantially higher in patients who are overtly immunosuppressed, such as those with B-cell malignancies, solid organ transplant, or hematopoietic cell transplant. It is likely that successful efforts to control and possibly even prevent melanoma using strategies that exploit the immune system will depend not only on the identification of the factors involved in tumor-host interactions but also in large part on tumor-specific immunogenic mutations that confer tumor cell oncogene addiction.
Immunosuppression, Tolerance Mechanisms, and Chronic Inflammation Induced by Melanoma
Although melanoma is not among the malignancies arising in the setting of chronic inflammation, its establishment is associated with the development of a local inflammatory microenvironment that initiates and maintains the suppressive or Th2 pattern of molecular and cellular components critical to its highly invasive and metastatic behavior. Many of these cells and molecules have been addressed earlier, and more detailed descriptions are beyond the scope of this article, but some of the important biological determinants and therapeutic targets include selected chemokines and their receptors (particularly CCL5 or regulated on activation, normal T cell expressed and secreted [RANTES], CCL11 or eotaxin, and CX chemokine 10 (CXCL10) or interferon gamma-induced protein 10 [IP-10]); VEGF; TGF-β; tumor necrosis factor alpha (TNF-α); and the cytokines IL-1, IL-6, and IL-8, all of which exert pleiotropic inflammatory effects on the surrounding cellular milieu. It is likely that the melanoma microenvironment exploits the inflammation amplifier, a circuit of cytokines, chemokines, neurotransmitters, growth factors/receptors, stromal elements, transcription factors, and inflammatory cells that work in concert to potentiate a state of local inflammation and tumor promotion and maintenance ( Fig. 5 ).
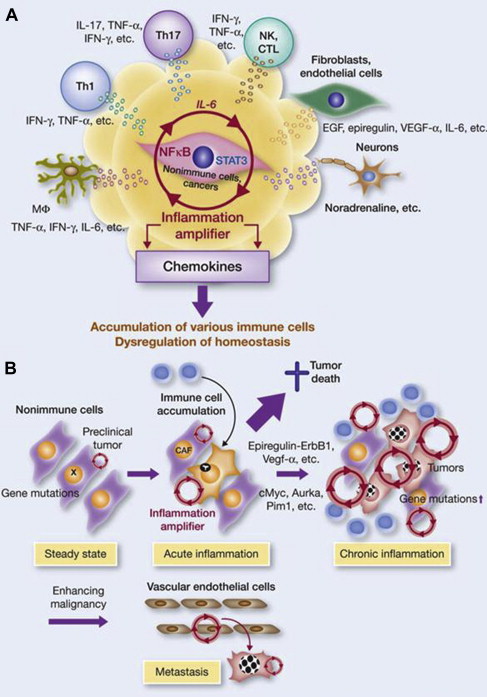
The immune tumor microenvironment has become the focus of intense scrutiny, because it has recently been shown to hold many of the secrets of tumor cell resistance to or escape from controlling mechanisms, which include but are not limited to those resulting from immune effector mechanisms. Although the current state of the field derived in large part from the study of phenotype and function of effectors and other inflammatory cells in the peripheral circulation, it is now clear that only by carefully dissecting and interrogating the tumor microenvironment can the precise impact of cell-cell and associated interactions be understood (as well as the role of cell populations that migrate in and out of sites of antigen presentation, including tumor). Further, these elements must be studied in several dimensions: histologic organization, quantitation of cell types, functional assays including serial analyses over time and in response to interventions, and sophisticated studies of the layers of omics reflecting genetics and gene expression in the various cell types comprising the tumor microenvironment. The results to date of such studies and the relative characteristics of the tumor immune contexture and the immunoscore are detailed earlier in this article ( Fig. 6 ).
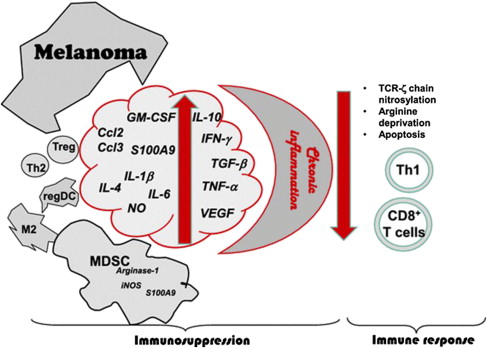
These observations have also provided corroboration of the immunoediting model of tumor–immune system interactions that features 3 broad phases: elimination of the tumor, equilibrium with the tumor, and escape by the tumor from immune control. These phases correspond with the experimental observations that more immunogenic tumors grow poorly in immunocompetent hosts and require host immunosuppression to grow well, whereas nonimmunogenic tumors grow well in immunocompetent hosts; a situation that is most closely related to human patients with melanoma ( Fig. 7 ).
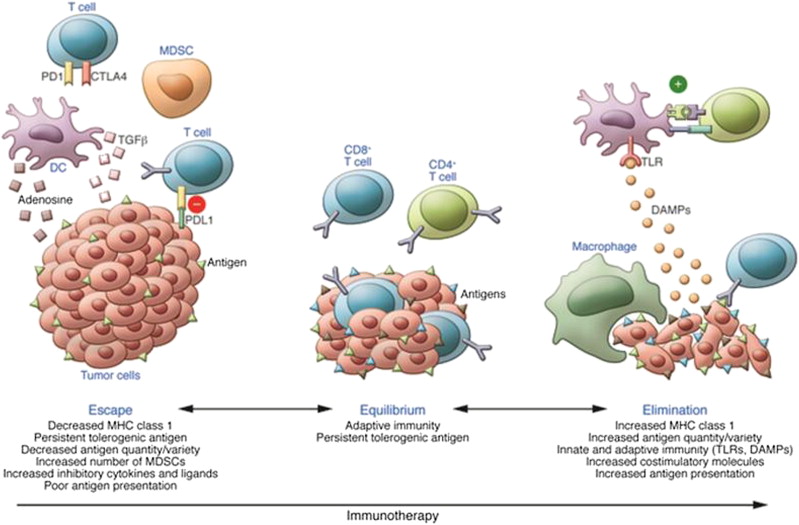
Experimental evidence for these phases is also consistent with the concept of 3 mechanisms that explain the failure of T-cell immune reactions to control cancer. The first 2 are likely the related results of antigen presented without adequate costimulation and consist of (1) self-tolerance (which can include the immunologic ignorance, whereby antigenic signals are delivered in a concentration or orientation insufficient to activate T cells but can be enhanced by higher concentration or exogenous inflammatory signals; a unique state of T-cell differentiation with a tolerance-specific gene program); and (2) anergy, defined as the inability to produce IL-2 or proliferate to antigen delivered under optimal conditions. The third phenomenon, which has been the focus of considerable investigation and some notable therapeutic successes over the last several years, is termed exhaustion, and refers to the state of immunologic inaction resulting from the downmodulatory response of T cells to chronic antigenic stimulation ( Fig. 8 ).
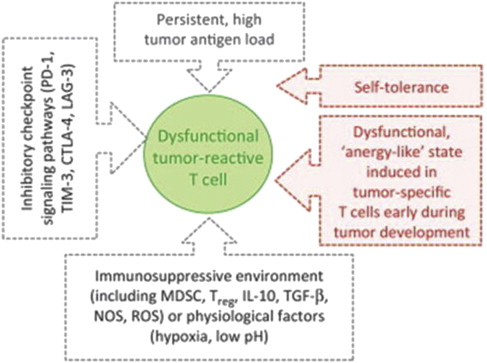
All of the factors associated with the immunosuppressed characteristics of the tumor microenvironment, including MDSC, T reg , IL-10, TGF-β, IDO, tumor-associated macrophage, and the low-pH/high–reactive oxygen species local chemical milieu are associated with the expression by tumor-infiltrating T cells of exhaustion markers such as PD receptor-1 and T-cell immunoglobulin and mucin domain-3 (TIM-3). Although maneuvers such as therapeutic lymphodepletion using chemotherapy can lead to the expansion of antigen-specific T cells and reverse tolerance, this approach alone is usually insufficient to induce tumor regression. However, many of the elements of the tumor immune microenvironment listed earlier are now amenable to therapeutic targeting with antibodies or small molecules and are currently under active investigation for melanoma and other malignancies, with encouraging results that are covered in detail elsewhere in this issue.
Background
Inflammation and Immunosurveillance in Melanomagenesis
Common knowledge holds that cutaneous melanoma is caused by harmful ultraviolet (UV) radiation, largely from intermittent scorching sunburns, particularly in childhood, an observation based on epidemiologic associations. The contribution of intentional exposure to erythema-inducing doses of UV wavelengths from tanning beds has been the focus of efforts to alter sun behaviors and enact legislation against access, particularly by minors, to tanning salons. It is also commonly thought that cancer immunosurveillance plays a large role in preventing or reducing the risk of invasive cancers, and there are plentiful examples in the literature for a strong association between immunosuppression or immunodeficiency and the occurrence of malignancy. However, despite a large and growing role for immunomodulatory therapies in advanced stages, melanoma is not one of the neoplasms occurring at significantly increased frequency among patients with compromised immunity. It is likely that the precise type and severity of immunodeficiency required to promote melanomagenesis or emergence of invasive, overt disease from a precursor or indolent disease occur only rarely.
Inflammation plays an important role in melanomagenesis, and inflammation is also a critical component of the liaisons between innate and adaptive immune responses, both of which have been shown to play roles in controlling tumors and protecting against new malignancy. Starting with the earliest stages of melanomagenesis, inflammation induced by UV light is associated with enhanced blood flow, vascular permeability, and damage to subcellular structures resulting from reactive oxygen species. In the earliest example of a tumor microenvironment reactive to oncogenic environmental factors, both the melanocytes and keratinocytes are induced by UV light to produce inflammatory substances that cooperate to prepare for tumor promotion and an immunosuppressive milieu and eventually the elaboration of growth factors that further support tumor growth, invasion, and metastasis.
Immunosuppression induced by UV light contributes to melanomagenesis via the reduction in the number of Langerhans cells; decreased antigen presentation; and elaboration of type 2 cytokines and other substances with suppressive effects, such as interleukin (IL)-4, IL-10, and prostaglandin-E2. UV light also stimulates the production of growth factors with tumor-promoting effects such as alpha-melanocyte–stimulating factor and platelet-activating factor. Neuropilin-1, a member of the vascular endothelial growth factor (VEGF) receptor family, contributes to the protumoral effects of a subset of regulatory T cells in melanoma, and its effects seem to be mediated by transforming growth factor beta (TGF-β) and to be synergistic with those of VEGF.
The role of chronic inflammation in melanomagenesis is less clear, although it has been suggested that solar elastosis, a consequence of prolonged rather than acute UV skin damage, may be protective and confer a more favorable prognosis in melanoma ; however, this observation may also be explained by some other favorable feature(s) of melanoma arising as a result of chronic UV damage rather than as a direct result of the solar elastosis. A promising therapeutic target is related to the phenomenon of T-cell immune exhaustion, caused in part by the interactions between ligands on the tumor cell as well as on other inflammatory cells, the programmed-death (PD)-1/PD-ligand (PD-L1) interaction, which is detailed later in this article and elsewhere in this issue by Naidoo and colleagues. Established melanoma is likely to represent successful tumor evolution through the 3 stages of immunoediting described by Schreiber and colleagues, beginning with elimination, evolving to equilibrium, and eventually resulting in escape. Also of likely immunotherapeutic importance are recent observations that the common molecular driver of melanoma and some other tumors, BRAF v600 E , confers alterations in melanocytes resulting in an immunosuppressive microenvironment that can be overcome by inhibitors of the enhanced mitogen-associated protein kinase (MAPK) pathway signaling ( Fig. 1 ). Whether other, less commonly occurring oncogenic drivers are similarly immunosuppressive remains to be shown, and the growing number of molecularly targeted inhibitors need to be carefully tested for their impact on cells of the immune system (both in the circulation and in the tumor microenvironment), because their on-target and off-target effects may be varied and unpredictable. Because molecularly and immunologically targeted therapies and the opportunities for combinatorial strategies are increasing rapidly, it is necessary to examine each component carefully in order to design regimens with the optimal therapeutic index.