Managing hematologic disorders in developing countries poses problems not encountered in Western societies. The clinical features of hematologic conditions may be modified by malnutrition, chronic bacterial infection, or parasitic illness. Iron deficiency is the major factor in anemia worldwide. Anemia is more common in the wet season when malaria transmission peaks. After anemia, eosinophilia is the next most common hematologic abnormality in children in the tropics. Infection with the human immunodeficiency virus can cause hematologic abnormalities. The pattern of distribution of primary disorders of the blood varies among populations and some disorders are unique to certain parts of the world.
Key points
- •
The diagnosis and management of hematologic disorders in developing countries pose a number of problems not encountered in advanced Western societies.
- •
The usual clinical features of hematologic disease may be modified to a varying degree by the coexistence of malnutrition, chronic bacterial infection, or parasitic illness.
- •
Iron deficiency is the major factor in anemia worldwide; numerous other diseases that exacerbate anemia are often operating in the setting of low body iron stores.
- •
The spectrum of hematologic complications associated with human immunodeficiency virus (HIV) and the high prevalence of infection make HIV testing an essential part of the investigation of cytopenias.
- •
The pattern of distribution of primary disorders of the blood varies considerably among different populations and some disorders are unique to certain parts of the world.
The diagnosis and management of hematologic disorders in developing countries pose a number of problems that are not encountered in advanced Western societies. Although all hematologic conditions can be seen in any population, their clinical features may be modified to a varying degree by the coexistence of malnutrition, chronic bacterial infection, or parasitic illness. Furthermore, many of the common killers, particularly in the tropics, produce their own complicated hematologic manifestations. It is often very difficult to define the clinical features and pathophysiology of a single hematologic disorder in this setting. It, therefore, follows that the study of hematologic disease in these populations presents a particular challenge for hematologists.
The human and material resources available to prevent, diagnose and treat hematologic disease vary widely, not only between countries, but also within countries. In many countries, particularly but not exclusively in newly industrialized countries, there is a long tradition of specialist training and a corresponding breadth and depth of expertise to treat patients with hematologic disease. However, there is a realization that in many parts of the world, there is a gap in knowledge and skills required not simply to treat hematologic problems, but also limited expertise to establish effective polices and training programs for clinical and laboratory hematology. The wider hematologic and scientific community and major donor agencies have responded with a series of initiatives to enhance training and the transfer of skills across the world.
In recent years, it has been possible to start to understand the pathogenesis of some of the hematologic manifestations of systemic disease in children in the Developing World. In this issue of Hematology/Oncology Clinics of North America , some of the progress that has been made in understanding and managing the wider aspects of hematology across the world or “global hematology” is reviewed.
Prevalence and multiple causes of anemia in the Developing World
Numerous surveys have been conducted to determine the prevalence of anemia in tropical populations. Until recently, it has been very difficult to interpret the results and compare one study with another. It is clear that in many populations the prevalence of anemia in preschool children is extremely high, and in some locations almost 100% of the population is affected. Twenty years ago, the attributable disability-adjusted life-years lost from anemia was estimated to be 35 million healthy life-years.
We now have much more geographically and etiologically defined data on the burden of anemia. Using publicly available data, Kassenbaum and colleagues estimated mild, moderate and severe anemia from 1990 to 2010 for more than 180 countries by sexes and well-defined age groups and attributed the cause of anemia using data from the Global Burden of Diseases, Injuries and Risk Factors 2010 Study and have summarized and updated the findings of this seminal study (in his article on The Global Burden of Anemia , in this issue).
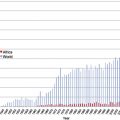
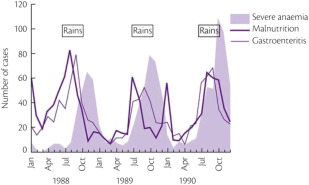
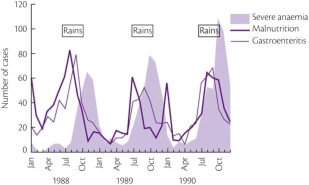
It is certainly difficult to determine the relative importance of different causes of anemia in the tropics. Most surveys have concentrated on only 1 mechanism, such as iron or folate deficiency. However, to get a true picture of the cause of anemia in a particular population, it is necessary to obtain consecutive data over a substantial period ( Table 1 ). For example, studies in The Gambia have shown that mean hemoglobin levels in children vary significantly at different times of the year; anemia is much more common in the wet season when malaria transmission is at its highest ( Fig. 1 ). This is also the time when diarrhea and malnutrition are most common because heavy rains after many dry months have profound effects on the community, sanitation measures are disrupted and food stores are at a low point in the annual cycle. Although these observations emphasize the multifactorial etiology of anemia, it is clear that iron deficiency, which affects at least 20% of the world’s population, is the major factor and that the numerous other diseases that may exacerbate anemia are often operating in the setting of low body iron stores.
| Age | Hemoglobin Concentration (g/dL) |
|---|---|
| Children, 6 mo–6 y | 11 |
| Children, 6–14 y | 12 |
| Adult males | 13 |
| Adult females (nonpregnant) | 12 |
| Adult females (pregnant) | 11 |
The body’s response to infection may also reduce iron stores and iron use. Hepcidin is regulated by proinflammatory mediators such as tumor necrosis factor and interleukin-6, which are increased in a wide variety of infections. However, this protective response may contribute to functional iron deficiency and anemia in endemic areas and the interrelationship between iron and infection is explored in more detail (see Pasricha S, Drakesmith H: Iron Deficiency Anemia – Problems in Diagnosis and Prevention at the Population Level , in this issue).
Iron deficiency and anemia are less common in groups that have persisted as hunter–gatherers or the pastoralists who eat blood and meat, such as the Masai in Kenya. In contrast, absorption of nonheme iron, except from breast milk, is comparatively restricted, and so iron deficiency is common in communities whose food is predominantly of vegetable origin. In addition, iron absorption is inhibited by fiber, phytates, phosphates, and polyphenols, which are all found at high levels in the largely vegetarian diets. Loss of iron from chronic hemorrhage secondary to hookworm infection or schistosomiasis further contributes to the high incidence of iron deficiency anemia in the developing world.
Infants of mothers with iron deficiency have low iron stores, their folate status at birth reflects that of their mother, and the folate content of breast milk is diminished by maternal deficiency and maternal malaria.
In many populations, anemia may be associated with folate deficiency. Again, the reasons are complex and multifactorial. Although intake of folate varies widely among different populations depending on the way in which food is prepared and the temperature at which it is cooked, it is clear that low intake is not necessarily the result of lack of folate in the diet. Loss of appetite associated with recurrent infections such as malaria or tuberculosis is the most important cause of folate deficiency in children and postinfective malabsorption is a particularly common cause of folate deficiency, especially in the Indian subcontinent. Folate deficiency can be exacerbated by the erythroid hyperplasia associated with chronic malarial infection or hemoglobinopathy.
Although nutritional vitamin B 12 deficiency is uncommon, Indian infants born to mothers with sprue (see Hematologic aspects of malabsorption in the tropics) who are fed breast milk or goat milk containing insufficient vitamin B 12 may be susceptible to megaloblastic anemia with locomotor complications during the early months of life. This syndrome, which is often fatal, seems to be complicated by a marked predisposition to infection.
Many of the population surveys on the prevalence of anemia in tropical countries have concentrated on 1 particular cause and other illness has not been assessed. Studies in Africa assessing body iron stores, folate levels and the presence of intercurrent infection indicate that chronic recurrent malaria, without other important complicating factors, is the major cause of anemia in these populations.
A major question is whether iron supplementation is the most effective way to prevent anemia in regions where malaria is highly endemic and this is discussed in more depth (see Pasricha S, Drakesmith H: Iron Deficiency Anemia – Problems in Diagnosis and Prevention at the Population Level , and Makani J, Roberts DJ: Hematology in Africa , in this issue). The optimal regime to combine malaria prophylaxis and iron supplementation has not been defined and this dilemma reflects a dichotomy between the optimal protective response to infection and the best physiologic environment for growth, development and recovery. A reduction in iron levels is part of natural host defense against infection, and there is long-standing debate about whether supplementation has adverse consequences, particularly on mortality from infectious diseases.
The outcome of a supplementation program may depend greatly on the pattern of endemic disease in the population and gathering good evidence from well-designed clinical trials to inform policy will undoubtedly be a major focus for future funding and work.
Prevalence and multiple causes of anemia in the Developing World
Numerous surveys have been conducted to determine the prevalence of anemia in tropical populations. Until recently, it has been very difficult to interpret the results and compare one study with another. It is clear that in many populations the prevalence of anemia in preschool children is extremely high, and in some locations almost 100% of the population is affected. Twenty years ago, the attributable disability-adjusted life-years lost from anemia was estimated to be 35 million healthy life-years.
We now have much more geographically and etiologically defined data on the burden of anemia. Using publicly available data, Kassenbaum and colleagues estimated mild, moderate and severe anemia from 1990 to 2010 for more than 180 countries by sexes and well-defined age groups and attributed the cause of anemia using data from the Global Burden of Diseases, Injuries and Risk Factors 2010 Study and have summarized and updated the findings of this seminal study (in his article on The Global Burden of Anemia , in this issue).
It is certainly difficult to determine the relative importance of different causes of anemia in the tropics. Most surveys have concentrated on only 1 mechanism, such as iron or folate deficiency. However, to get a true picture of the cause of anemia in a particular population, it is necessary to obtain consecutive data over a substantial period ( Table 1 ). For example, studies in The Gambia have shown that mean hemoglobin levels in children vary significantly at different times of the year; anemia is much more common in the wet season when malaria transmission is at its highest ( Fig. 1 ). This is also the time when diarrhea and malnutrition are most common because heavy rains after many dry months have profound effects on the community, sanitation measures are disrupted and food stores are at a low point in the annual cycle. Although these observations emphasize the multifactorial etiology of anemia, it is clear that iron deficiency, which affects at least 20% of the world’s population, is the major factor and that the numerous other diseases that may exacerbate anemia are often operating in the setting of low body iron stores.
| Age | Hemoglobin Concentration (g/dL) |
|---|---|
| Children, 6 mo–6 y | 11 |
| Children, 6–14 y | 12 |
| Adult males | 13 |
| Adult females (nonpregnant) | 12 |
| Adult females (pregnant) | 11 |
The body’s response to infection may also reduce iron stores and iron use. Hepcidin is regulated by proinflammatory mediators such as tumor necrosis factor and interleukin-6, which are increased in a wide variety of infections. However, this protective response may contribute to functional iron deficiency and anemia in endemic areas and the interrelationship between iron and infection is explored in more detail (see Pasricha S, Drakesmith H: Iron Deficiency Anemia – Problems in Diagnosis and Prevention at the Population Level , in this issue).
Iron deficiency and anemia are less common in groups that have persisted as hunter–gatherers or the pastoralists who eat blood and meat, such as the Masai in Kenya. In contrast, absorption of nonheme iron, except from breast milk, is comparatively restricted, and so iron deficiency is common in communities whose food is predominantly of vegetable origin. In addition, iron absorption is inhibited by fiber, phytates, phosphates, and polyphenols, which are all found at high levels in the largely vegetarian diets. Loss of iron from chronic hemorrhage secondary to hookworm infection or schistosomiasis further contributes to the high incidence of iron deficiency anemia in the developing world.
Infants of mothers with iron deficiency have low iron stores, their folate status at birth reflects that of their mother, and the folate content of breast milk is diminished by maternal deficiency and maternal malaria.
In many populations, anemia may be associated with folate deficiency. Again, the reasons are complex and multifactorial. Although intake of folate varies widely among different populations depending on the way in which food is prepared and the temperature at which it is cooked, it is clear that low intake is not necessarily the result of lack of folate in the diet. Loss of appetite associated with recurrent infections such as malaria or tuberculosis is the most important cause of folate deficiency in children and postinfective malabsorption is a particularly common cause of folate deficiency, especially in the Indian subcontinent. Folate deficiency can be exacerbated by the erythroid hyperplasia associated with chronic malarial infection or hemoglobinopathy.
Although nutritional vitamin B 12 deficiency is uncommon, Indian infants born to mothers with sprue (see Hematologic aspects of malabsorption in the tropics) who are fed breast milk or goat milk containing insufficient vitamin B 12 may be susceptible to megaloblastic anemia with locomotor complications during the early months of life. This syndrome, which is often fatal, seems to be complicated by a marked predisposition to infection.
Many of the population surveys on the prevalence of anemia in tropical countries have concentrated on 1 particular cause and other illness has not been assessed. Studies in Africa assessing body iron stores, folate levels and the presence of intercurrent infection indicate that chronic recurrent malaria, without other important complicating factors, is the major cause of anemia in these populations.
A major question is whether iron supplementation is the most effective way to prevent anemia in regions where malaria is highly endemic and this is discussed in more depth (see Pasricha S, Drakesmith H: Iron Deficiency Anemia – Problems in Diagnosis and Prevention at the Population Level , and Makani J, Roberts DJ: Hematology in Africa , in this issue). The optimal regime to combine malaria prophylaxis and iron supplementation has not been defined and this dilemma reflects a dichotomy between the optimal protective response to infection and the best physiologic environment for growth, development and recovery. A reduction in iron levels is part of natural host defense against infection, and there is long-standing debate about whether supplementation has adverse consequences, particularly on mortality from infectious diseases.
The outcome of a supplementation program may depend greatly on the pattern of endemic disease in the population and gathering good evidence from well-designed clinical trials to inform policy will undoubtedly be a major focus for future funding and work.
Hematologic changes associated with specific infections in the tropics
Many, if not all, systemic infections are associated with hematologic changes and the common parasitic infections that are widespread across the tropics have a wide variety of hematologic manifestations that may be a presenting feature or form part of a more complex clinical picture.
Malaria
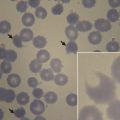
Malaria is the most important parasitic illness of humans and because of its intraerythrocytic life cycle the malarial parasite is particularly prone to cause hematologic manifestations. Anemia caused by ineffective erythropoiesis and increased red blood cell destruction is a common, debilitating and often serious manifestation of many forms of malaria (see Roberts DJ, Field S, Delaney M, et al: Problems and Approaches for Blood Transfusion in the Developing Countries , in this issue).
Visceral Leishmaniasis (Kala-azar)
Leishmaniasis is an infection caused by intracellular protozoan parasites transmitted by various species of sandflies. The important hematologic manifestations of leishmanial infection are found in the visceral forms. Severe neutropenia may also occur in young children, and this, together with dyserythropoietic changes in the marrow, marked erythrophagocytosis and bizarre mononuclear infiltrates, may cause confusion with leukemia.
Schistosomiasis
The schistosomes are a group of trematodes that cause major health problems in many parts of the developing world. The most consistent hematologic changes are found in association with Schistosoma mansoni infection. The acute phase eosinophilia. As the disease progresses, there may be massive hepatosplenomegaly associated with anemia, neutropenia and thrombocytopenia, Schistosoma haematobium iron deficiency anemia and/or features of the anemia of chronic disorders.
Trypanosomiasis
Trypanosomal infections are a major cause of ill health throughout the world. American trypanosomiasis is a zoonosis caused by Trypanosoma cruzi , which is transmitted to humans in South America by blood-sucking insects. The hematologic changes in Chagas’ disease are nonspecific. In the acute phase, mild anemia and lymphocytosis may occur. African trypanosomiasis is caused by Trypanosoma brucei and, during the acute phase, normochromic normocytic anemia is present. Occasionally, patients have hemorrhagic manifestations and evidence of disseminated intravascular coagulation.
Hookworm
It has been estimated that more than 900 million people are infected with hookworms, which may cause iron deficiency anemia. A full discussion of the presentation and pathology of anemia associated with infection is given elsewhere (see Roberts DJ: Hematologic Changes Associated with Specific Infections in the Tropics , in this issue).
Complex Hematology of Childhood Human Immunodeficiency Virus Infection in the Developing Countries
Human immunodeficiency virus (HIV) infection provides yet another example of the complex pathogenesis of hematologic problems in the tropics. The virus can infect hematopoietic precursors, as well as bone marrow macrophages and stromal fibroblasts, an observation that may explain the marked dyserythropoiesis that is observed commonly in the bone marrow of affected children; however, these changes may also be complicated by opportunistic infection, malnutrition and drug toxicity. Anemia with a low reticulocyte count and a microcytic hypochromic blood picture is common and may reflect both coexistent iron deficiency and the anemia of chronic disorders. HIV may cause immune thrombocytopenia early in the course of the disease, but this is not regarded as an adverse prognostic feature. Thrombocytopenia may also be accompanied by microangiopathic hemolytic anemia as part of thrombotic thrombocytopenic purpura, which in the context of HIV infection, responds well to plasma infusion. Later, in addition to the progressive lymphopenia that characterizes acquired immunodeficiency syndrome, both reduced production and increased cellular clearance may contribute to neutropenia and thrombocytopenia. Finally, advancing immunodeficiency contributes to a greatly increased risk for lymphoid neoplasms, particularly lymphoma.
The wide spectrum of hematologic complications associated with HIV and the high prevalence of infection make HIV testing an essential part of the investigation of cytopenia in most tropical areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree