Fig. 1.1
Current way of developing anticancer drugs
Since recently, advances in high-throughput technologies have allowed depicting most druggable molecular alterations for an affordable cost in a time frame compatible with clinical practice. Despite the caveats associated with histology-independent drug development mentioned above, the question whether precision medicine based on the molecular profiling of the tumor of cancer patients would still improve their outcome has arisen and led to set up clinical trials addressing this question (Fig. 1.2). These clinical trials evaluate a treatment algorithm instead of drug efficacy. These studies are associated with numerous challenges that are later discussed.
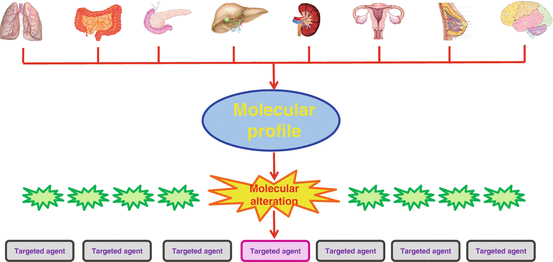

Fig. 1.2
Concept of precision medicine
1.2 Personalized Medicine Trials
One fundamental requirement in these precision medicine trials is that the treatment algorithm is not modified during the study. These trials include nonrandomized trials that usually use patients as their own control to assess efficacy, and randomized trials that address various questions. All these trials have been set up in the metastatic setting, either in patients with refractory cancers or as maintenance therapy. If the precision medicine concept is validated, it will have to be evaluated at earlier stages of the disease.
Von Hoff’s study was the first published histology-independent clinical trial using tumor molecular alterations to select treatment (von Hoff et al. 2010). Patients with any type of recurrent and/or metastatic cancer that was refractory to standard of care had selected molecular alterations analyzed using immunohistochemistry (IHC), FISH, and oligonucleotide microarray gene expression assays. Based on the detected molecular alterations, a drug or drug combination was prescribed. Efficacy was assessed using each patient as his own control by determining the time of disease control on the treatment guided by the molecular profile compared to the time of disease control on the last treatment. Disease control was assessed using well-established criteria to measure progression-free survival (PFS). Eighteen of the 66 treated patients (27 %) had a ratio of the PFS on matching targeted treatment to the PFS under the last previous treatment >1.3, which was considered a positive result. More recently, the WIN Consortium launched the WINTHER trial (NCT01856296) which is similar to von Hoff’s study in its concept (http://www.cancer.gov/clinicaltrials/search/view?cdrid=749710&version=HealthProfessional&protocolsearchid=12176033). This trial is open to patients with any kind of refractory advanced cancer. Two samples are taken from the patient, the first one from a metastatic site and the second from adjacent normal tissue. Druggable molecular alterations are first investigated from the tumor sample using next-generation sequencing (NGS) for mutations screening and comparative genomic hybridization (CGH) array for gene copy number variations. If a druggable molecular alteration is identified, patients are either guided to a phase I clinical trial with an agent presumably matching the molecular alteration or are being prescribed an already approved MTA off-label. If no druggable molecular alteration is detected, data from tumoral RNA and RNA from normal adjacent tissue are analyzed in order to identify gene expression profiles that can orient the patient to the best therapy. Both treatment arms will be analyzed separately using PFS ratio as a primary end point. The main advantage of this trial is that all included patients will be treated. Given the multiple tumor types included in these nonrandomized trials, one way to evaluate treatment efficacy has been to use patients as their own controls assessing the PFS ratio. The main criticism to the use of the PFS ratio as a primary end point in these studies is the assessment of PFS on the last therapy outside of the clinical trial. In addition, the underlying assumption behind this end point is that the natural history of disease is linear over time, in other words that the two PFS are correlated, which might not be true. For these reasons, the use of randomization has been suggested (Doroshow 2010).
The SHIVA trial (NCT01771458) was a proof-of-concept randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional chemotherapy in patients with any type of cancer that was refractory to standard of care (Le Tourneau et al. 2012a). The primary end point was PFS. Given the heterogeneity in terms of prognosis of patients with various tumor types, the trial was stratified on (1) the patient’s prognosis using the Royal Marsden Hospital prognostic score for refractory cancer patients (Arkenau et al. 2009) and (2) the signaling pathway, the selected molecular alteration belongs to. Molecular alterations were evaluated on a tumor sample from a metastatic site using NGS for mutations screening, CytoScan HD for gene copy number variations, and IHC for estrogen, progesterone, and androgen receptor expression analyses. Only marketed MTAs were used in this trial according to a prespecified treatment algorithm. Eleven MTAs were available within the clinical trial, whereas conventional chemotherapy was prescribed at the physician’s discretion in the control arm. Crossover was proposed in both arms at disease progression, allowing the evaluation of tumor growth kinetics on both treatments for each patient (Le Tourneau et al. 2012b). Physicians were being told the molecular alteration of interest for their patient only at the time the patients were about to be treated in the experimental arm. Feasibility results on the first 100 included patients have shown that biopsies are safe and that at least one molecular alteration was detected in 40 % of patients allowing them to be randomized (Le Tourneau et al. 2014). Ancillary studies include the evaluation of the ability of circulating DNA to predict treatment efficacy or resistance, as well as a medico-economic evaluation of the experimental strategy. Efficacy results showed a differential effect depending on the signaling pathway (Le Tourneau et al. 2015). While the overall result was negative, patients treated with MTAs targeting the RAF/MEK pathway had a longer PFS in the experimental arm (3.7 months as compared to 2.0 months). This suggests that the precision medicine approach might be valid in this subgroup. This will be evaluated in the SHIVA02 trial that should start in 2016.
The MPACT trial is an ongoing randomized phase II trial led by the National Cancer Institute that includes as the same patient population the SHIVA trial (Kummar ASCO meeting 2013). A tumor sample of a metastatic site is also mandatory. Molecular alterations are detected using similar technologies as in the SHIVA trial. Patients are randomized between therapy matching the detected molecular alteration and therapy not matching the detected molecular alteration. Crossover is proposed at disease progression for patients randomized in the nonmatching treatment arm. Although it might be difficult for patients to accept the randomization in the nonmatching treatment arm, this design is the only one that evaluates solely the treatment algorithm. Accrual has started in 2014.
The MOST trial (EudraCT: 2012-004510-34) is a randomized discontinuation trial for patients who have progressed on first-line treatment for a recurrent and/or metastatic cancer led by the Centre Léon Bérard in Lyon (http://www.cancer-lyric.com/programme/programme-1/). Molecular alterations are identified on a sample from either a metastatic site or the primary tumor using similar technologies as in the previous trials. Patients are treated during 3 months with one of the five available already marketed MTAs. Responding patients will continue on therapy, while progressive patients will be taken off study. Patients with stable disease are randomized between treatment continuation and discontinuation for 2 months. The MOST trial will provide a more accurate evaluation of efficacy than a single-arm study by deciphering between disease stabilization related to the natural history of the disease and disease stabilization related to a cytostatic effect of molecularly targeted therapy. The trial has started in 2014.
The SAFIR 02 trials are tumor-specific randomized trials evaluating maintenance therapy (Andre et al. 2014). SAFIR 02 Breast includes patients with HER-2-negative and estrogen receptor-positive recurrent and/or metastatic breast cancer who have not progressed after four to eight cycles of first- or second-line chemotherapy, while SAFIR 02 Lung includes patients with EGFR and ALK wild-type recurrent and/or metastatic lung cancer who have not progressed after four cycles of first-line platinum-based chemotherapy. All patients have a tumor sample taken from a metastatic site in order to seek molecular alterations using the same technologies. Patients are randomized between an MTA from AstraZeneca matching the detected molecular alteration and maintenance chemotherapy. The primary end point is PFS. These trials have opened in 2014 as well. These trials evaluate the utility of a treatment algorithm for selecting maintenance therapy following first-line therapy in recurrent and/or metastatic luminal breast cancer and lung cancer not eligible for molecularly targeted therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree