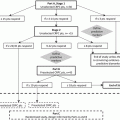
Fig. 1.1
Management-based dynamic progression of prostate cancer and the mCRPC state. ADT androgen deprivation treatment
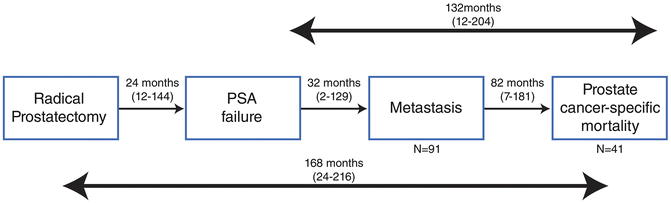
Fig. 1.2
Survival of metastatic prostate cancer patients diagnosed in the context of a close follow-up after biochemical relapse: The Johns Hopkins Experience (from Makarov et al. [4])
An increasing proportion of patients with relapsed prostate cancer receive androgen deprivation prior to the development of metastatic disease and eventually demonstrate evidence of advancing disease initially manifested by rises of serum PSA levels without any other clinical/radiological evidence of disease. This subset of patients is classified as non-metastatic castration resistant disease. The clinical course of these patients is extremely variable. Factors that could account for the outcome in the castration resistant M0 patients include: initial criteria for initiation of ADT (PSADT, Gleason’s score, time from local treatment to evidence of biochemical recurrence), response to the initial hormonal therapy, PSADT at recurrence, and PSA level in the castrate state [5].
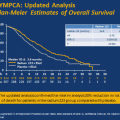
Less than 5 % of patients diagnosed with prostate cancer today demonstrate clinical evidence of distant metastasis at presentation. The survival of men with newly diagnosed metastatic prostate cancer has changed significantly during the past two decades and this is illustrated by the differences in outcome of patients enrolled onto clinical trials over the past two decades. From 1985 to 1986 (pre PSA era) the NCI sponsored an intergroup study comparing leuprolide acetate with or without flutamide in patients with newly diagnosed, hormone naïve prostate cancer. The median survival in the combination arm (best arm) was 31 months. From 1989 to 1993 the Southwest Oncology Group (SWOG) conducted a trial in 1,387 men with newly diagnosed prostate cancer treated with bilateral orchiectomy with or without the antiandrogen flutamide (SWOG8894, double-blinded placebo-controlled trial) which resulted in no significant differences between arms and the overall median survival in these patients was 33 months [4]. From 1995 to 2009 the SWOG conducted a study in the same patient population (SWOG 9936) which employed in one the arms a GnRH analogue with bicalutamide, median survival data on patients treated with continuous ADT was 49 months [6]. The risk of death observed in SWOG 9936 was significantly lower compared to SWOG 8894 (HR 0.77–0.6–0.8–p < 0.0001) suggesting a 30 % reduction of risk of death [6] (Fig. 1.3). The proportion of men that present with baseline unfavorable prognostic factors (extent of disease, presence/absence of pain and baseline PSA value) on is significantly lower in SWOG 9936 compared to the SWOG 8894 [6].
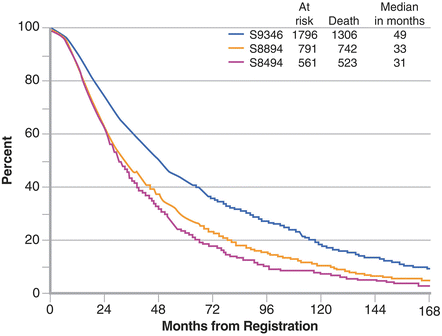

Fig. 1.3
Kaplan–Meier survival of newly diagnosed metastatic, hormone naïve, prostate cancer patients in three studies conducted by the Southwest Oncology Group (reprinted from Tangen et al. [6])
The m CRPC survival figures have also evolved in a similar fashion based on clinical trials data. The median survival of patients entered onto the two randomized studies completed most recently in patients with mCRPC in the “pre-chemotherapy space” is in excess of 30 months. This compares to approximately 19 months in patients with “hormone-refractory disease” reported 10 years ago in the TAX-327 docetaxel study and less than 12 months in the phase 3 mitoxantrone studies conducted in the 1990s.
An important chapter of this book involves a careful description of the extensive research around newer imaging modalities which will undoubtedly enhance our ability to diagnose metastasis at an earlier stage and define the tumor burden more adequately than conventional modalities. In Chap. 4 Cho, Dianat, and Macura describe newer modalities of imaging which may provide new opportunities for treatment besides diagnostics. The authors point out that while earlier diagnosis and treatment of metastasis has not necessarily been proven to extend survival it is likely that identification of patients with early metastasis offers an excellent opportunity for studying this subject. Furthermore, new imaging modalities may facilitate the conduct of clinical by introducing new endpoints and allow for a more adequate assessment of metastatic involvement, quantification of the metastatic burden, and possibly introducing new criteria for response based on functional status of metastatic lesions.
The Biology and Treatment
Prostate cancer growth is driven primarily by androgen receptor (AR). Increased understanding of the biology of AR signaling and downstream AR regulated genes provided the opportunity for therapeutic targeting and resulted in the development of newer compounds that have been shown to improve survival of m CRPC patients prior and after chemotherapy and hence prove the hypothesis that AR signaling remains an important regulatory factor in the growth of prostate cancer even after adequate gonadal suppression. In Chap. 3, Nelson and Pienta review the current status and future directions of basic research of molecular mechanisms involved in prostate cancer growth. It is expected that an increase in the understanding of the biology of prostate cancer will continue to provide new opportunities for treatment. The molecular heterogeneity of prostate cancer has long been recognized and emphasized in recent “warm autopsy studies.” It is becoming increasingly clear that adequate biological characterization of the disease will allow for a more logical selection of patients and facilitate individualized treatment strategies. Correlational studies evaluating molecular biomarkers, clinical outcomes such as response and toxicities are likely to become hallmarks of drug development in this disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree