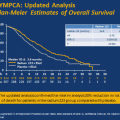
Targeted treatment combined with docetaxel
Phase I docetaxel combination trial in CRPC, N
Any unexpected safety signals?
Pharmacodynamic studies
Phase II docetaxel-combination trial in CRPC design (N)
Statistical plan
Primary and secondary endpoints
Outcome
Comments
Phase III docetaxel combination design (N)
Statistical plan
Primary and secondary endpoints
Outcome
Comments
References
Bevacizumab
NA
Single arm phase II, N = 20
No sample size calculation done
Response (biochemical and radiological). Secondary endpoints: Toxicity, PFS, OS
11/20 (55 %) confirmed PSA declines ≥ 50 %. 3 PR
Docetaxel 60 mg/m2, Bevacizumab 10 mg/m2
Randomized double blind placebo-controlled N = 1,050
86 % power to detect an increase in mOS of 24 (DP + B) vs 19 m (DP), alpha 0.05
OS, secondary: 50 % PSA decline, PFS, ORR, toxicity
mOS 22.6 (DP + B) vs 21.5 m (DP), HR 0.91, (95 % CI 0.78-1.05)
Increased treatment related deaths 4 vs 1.2 % p = 0.05; PFS improved 9.3 vs 7.5 m p < 0.001; ORR improved 49.4 vs 35.5 %, p 0.0013
Aflibercept
NA
Randomized double blind placebo-controlled N = 1,224
Powered to detect a HR of 0.8 with 90 % power, alpha 0.044
OS, secondary: PFS, PSA PFS, ORR, safety, pain response, pain PFS
22.1 (DP + A) vs 21.2 m (DP), HR 0.94, (95 % CI 0.82-1.08)
Significantly more G3-4 GI-toxicity (30 vs 8 %), hemorrhagic events (5.2 vs 1.7 %) and treatment related fatal events (3.4 vs 1.5 %)
Atrasentan
N = 12
Increased rate of hematological toxicity (neutropenic fever) in combined phase I/II study (21 %)
Bone alkaline phosphatase, urinary N-telopeptides
N = 19 (expansion cohort)
Max. 24 pt expansion cohort, improvement of PSA response rate from 40 to 65 %, type I error 0.05, power 0.86
PSA decline, Soft-tissue response
Confirmed PSA declines ≥50 % in 22.5 % pts, confirmed PSA declines ≥30 % in 35 % pts. Soft-tissue responses in 2/13 pts (15 %)
Prednisone was not given in combination with docetaxel and may account for lower PSA decline rates. MTD was not clearly defined, a dose of docetaxel of 70-75 mg/m2 was recommended. Overall survival 17.6 m (19.2 in TAX327 study)
D vs D + Atrasentan, 1:1; N = 994
25 % increase in PFS (6.0 vs 7.5 m) and in OS (18 vs 22.5 m)
Co-primary endpoints PFS and OS
Trial halted for futility after third interim analysis. PFS 9.2 vs 9.1 m (HR 1.02, 95 % CI 0.89–1.16). OS 17.8 vs 17.6 m (HR 1.04, 95 % CI 0.90–1.19)
Atrasentan monotherapy Phase III trial in CRPC did also not meet the primary endpoint
Zibotentan
N = 6
No DLTs, all 3 pts in the 15 mg Zibotentan group had ≥ G3 AE (G3 neutropenia, G3 hypertension, G3 gastrointestinal hemorrhage)
NA
Randomized phase II, N = 31 (20 DP + Zibotentan vs 11 DP)
Increase in PSA response rate from 45 % (TAX327) to 65 %, alpha 0.2, power 75 %
PSA response
PSA response 85 vs 73 %, ORR 22 vs 17 %
Urinary NTx stable in D + Zibotentan group, bone alkaline phosphatase and procollagen type I N propeptide (PINP) reduced in both groups. c-Terminal cross-linking telopeptides unchanged.
Randomized double blind placebo-controlled N = 1,052
Powered to detect a HR of 0.75 with 90 % power, alpha 0.05
OS, secondary endpoints: pain PFS, PFS, SRE, PSA PFS and PSA response rate
mOS 20 vs 19.2 m (HR 1.00, 95 % CI 0.84-1.18)
No differences in secondary endpoints. Cardiac failure 5.6 vs 1.7 %
Dasatinib
N = 16
No DLTs, MTD not reached
Urinary N-telopeptide, bone alkaline phosphatase
N = 30 (single arm phase II)
Not given
Not given
PSA declines ≥ 50 % for ≥ 6 in 26 of 46 patients (57 %). Soft-tissue partial responses 18/30 evaluable pts (60 %)
Double blind, placebo-controlled, 1:1; D + P vs D + P + Dasatinib; N = 1,522
NA
OS; ORR, time to first skeletal-related event (TFSRE), time to prostate-specific antigen progression (TPSAP), urinary N-telopeptide (uNTX) reduction, pain reduction, progression-free survival (PFS), and safety
21.5 vs. 21.2 m; HR 0.99; p = 0.90
Modest delay in SREs
GVAX
No docetaxel combination study, monotherapy in CRPC Phase I/II (N = 80)
NA
N = 600
NA
OS
12.2 (DP + GVAX) vs 14.1 m (DP) (HR 1.7, 95 % CI 1.15-2.53)
Trial terminated early due to an imbalance in deaths in the experimental arm.
Lenalidomide
N = 34
G4 neutropenia (2 pts) and febrile neutropenia (1 pt)
NA
NA
Not performed
Double blind, placebo-controlled, 1:1, D + P vs D + P + Lenalidomie; N = 1,059
NA
Primary: OS. Secondary PFS, ORR, safety
OS NR (D + P) vs 17.8 m (D + P + L) (HR 1.53, 95 % 1.17-2.0), PFS NR (D + P) vs 10.4 m (D + P + L), (HR 1.32, 95 % CI 1.05-1.66)
Median number of treatment cycles 8 (D + P) vs 6 (D + P + L), rate of febrile neutropenia 12 %
Calcitriol
NA
N = 37 (Docetaxel 36 mg/m2 for 5 weeks of a 6 week cycle, calcitriol 0.5 μg/kg)
20 % increase in PSA response rate from 40 to 60 %, alpha 0.05, power 80 %
Confirmed PSA decline ≥50 %. Secondary endpoints: ORR, PFS, OS
PSA response 81 %, ORR 53 %. mOS 19.5 m
[35]
N = 250, double blind randomized. Docetaxel 36 mg/m2 for 3 weeks of 4 cycle, DN-101 45 μg (1 day before chemotherapy)
20 % increase in PSA response rate from 45 to 65 %, alpha 0.045, power 85 %
Confirmed PSA decline ≥50 %. Secondary endpoints: ORR, PFS, OS
PSA responses 58 vs 49 %
mOS not reached for DP + DN101 estimated 24.5 vs 16.4 m (DP), (HR 0.7, 95 % CI 0.48-1.04)
Randomized open label phase III, N = 953
Powered to detect a HR of 0.78 for OS, power 90 %, alpha 0.05
Primary: OS. Secondary: safety, PFS, SRE-free survival
Trial halted for futility. OS 17.8 m (DP + DN-101) vs 20.2 m (p = 0.002)
Significantly more toxicity leading to Docetaxel dose modification 31 vs 15 %
Custirsen (OGX-011)
40 pts, 8 cohortsq
At OGX-011 640 mg and Docetaxel total 9 pts 3 DLTs (G4 mucositis, G3 fatigue, G4 neutropenia 6 days)
Clusterin expression in mononuclear cells decreased in all pts
Randomized phase II (N = 82 pts)
90 % to detect difference in PSA response rate of 60 vs 40 %, alpha 0.1
Confirmed PSA decline ≥50 %. Secondary endpoints: ORR, PFS, OS, changes in serum clusterin
PSA declines ≥50 %: 58 vs 54 %. ORR 19 vs 25 %
mOS 23.8 vs 16.9 m (HR 0.61, 95 % CI 0.36-1.02). Median decrease in serum clusterin at end of cycle 1 in DP + OGX-011: 26 % vs increase in 0.9 % on DP alone
Double blind, NOT placebo-controlled, N = 1,000
NA
OS. Secondary: PFS, safety, ORR, PSA response rate
NA
NA
[38]
Strontium +/− Zoledronic acid
NA
N = 200 (4 arms: DP vs DP + Zoledronate vs DP + Sr89 vs DP + SR89 + Zoledronate)
NA
Feasibility and safety
No safety issues identified, trial moved into phase III part
NA
N = 757; patients randomized to six cycles of DP: alone; with ZA; with a single dose of Sr89 after cycle 6 or both
Power 90 %, alpha 0.05
Co-primary endpoints: bone-clinical PFS (SRE, death from any cause, bone pain progression). Secondary EP: OS
Clinical PFS DP + Sr89 9.8 vs 8.8 m (DP), HR 0.8, (95 % CI 0.66-0.97)
mOS DP + SR89 16.8 vs 16.4 m (DP)
[39]
The results of the Phase III trials were not only disappointing because they were negative but in the cases of lenalidomide, GVAX, and calcitriol the median OS was significantly worse in the combination arm compared to standard docetaxel plus prednisone and for bevacizumab and aflibercept increased rates of toxicity and treatment related deaths were reported. The lessons learnt from the negative Phase III docetaxel-combination trial are mainly that more time and effort should be spent on the design and conduct of the early Phase I/II trial and to have a clear activity signal based on a biological rationale before launching into large and costly Phase III trials.
The Challenges of Performing Trials in CRPC
Survival Endpoints
With the approval of several novel agents in the last 4 years, patients are likely to be exposed to several survival-prolonging treatments, impacting OS benefits from an experimental therapy tested as an early line of treatment. A median OS benefit for a novel agent may therefore no longer be an achievable endpoint. With the COU-AA-302 trial pre-chemotherapy abiraterone was only licensed based on the proven survival benefit post-docetaxel and the significant improvement in the co-primary and all secondary endpoints. However, orteronel (TAK-700) may be the first casualty of the increased access to active post-trial therapies. The changing landscape of drug development in CRPC therefore urgently requires validation of intermediate endpoints.
Surrogacy Endpoints
In order to fulfill the criteria of surrogacy a biomarker must meet several prospectively defined criteria (termed the Prentice Criteria) and must be validated across multiple trials of different anticancer treatments [41]. At present there are no surrogate endpoints for OS in CRPC. Decreases in circulating tumor cell (CTC) counts and the combination of CTCs and LDH have shown promise as survival surrogates [42, 43].
Prognostic Markers
At least 20 baseline (pre-treatment) and eight on-treatment factors have been shown to have prognostic significance in CRPC patients [44]. These factors were combined into nomograms that predicted survival for chemotherapy-naïve CRPC patients [45–48]. These nomograms were developed before the introduction of the novel treatments and require updating for contemporary CRPC populations [49].
Increasing use of molecular characterization technologies may soon allow CRPC patients to be stratified within trials, or for development of niche therapies targeting key mutations in subsets of CRPC patients. Although these approaches are likely to improve response rates, challenges of molecular heterogeneity within tumors and patients, as well as parallel signaling pathways within cancer cells, will need to be addressed moving forward.
Tissue Acquisition
Translational studies often require tissue and mandating available blocks or slides at study entry is one method for protecting the validity of these important exploratory objectives. Although prostate cancer most commonly metastasizes to bone, making biopsy more challenging, the ability to maximize opportunities for prostate cancer patients to participate in both cancer-specific and solid tumor Phase I studies should encourage clinicians to pursue tissue acquisition whenever possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree