There has been a significant steady increase in the number of thyroid surgeries performed annually in the United States,1 escalating existing pressures to maintain a low rate of complications in this surgical setting.
Recurrent laryngeal nerve (RLN) injury is an unfortunate complication of thyroid surgery. Unilateral vocal cord paralysis may create changes in voice, which if severe may impose vocational limits or loss, lead to dysphasia, or culminate in pulmonary complications due to aspiration.2 Life-threatening loss of airway, with a need for tracheostomy, is the consequence of bilateral vocal cord paralysis.3
The external branch of superior laryngeal nerve (EBSLN) is vulnerable to injury when dissecting the superior pole of the thyroid gland. Injury to EBSLN may cause paralysis of cricothyroid muscle, which is difficult to detect by endoscopic means. Although EBSLN injuries affect the voice variably and may be minor, any such deficit is potentially devastating to professional vocalists and others whose livelihood is similarly susceptible.
Intraoperative visual identification of nerves is the method traditionally used to avoid RLN and EBSLN injuries during thyroid surgery. Still, a functional nerve cannot be assured by gross visualization alone. Neural monitoring adds a novel functional dynamic to customary protocol. We do not consider the use of intraoperative nerve monitoring as the standard of care to decrease the risk of possible complications related to the nerve injury. Herein, the benefits of intraoperative nerve monitoring are discussed, detailing a multifaceted approach that extends beyond basic visual inspection.
Intraoperative nerve monitoring (IONM) technologies were first reported in 1965 by Shedd and Durham4 as a novel method to reduce the risk of RLN injury. In 1970, the use of intramuscular vocal cord electrodes was described by Basmajian.5 In addition, there is a long history of IONM in a variety of head-and-neck and skull-base procedures, where such monitoring is associated with improved outcomes.
Although visual identification of RLN decreases the rate of permanent RLN injury, it remains the most common source of medicolegal litigation following thyroid surgeries.5 Due to the morbidity inflicted, awards to plaintiffs for bilateral vocal cord paresis may amount to millions of dollars.3 However, there is no evidence that intraoperative nerve monitoring is associated with a decreased risk of RLN injury. Dralle et al6 suggested that conducting a study with the typical rates of nerve injury would need millions of patients to be statistically powerful.
It is prudent to maintain detailed records of pre- and postoperative patient visits, including pivotal discussions. Preoperative emphasis on RLN injury as a potential complication is especially important, as well as documenting this risk clearly in informed consent. If IONM is used, operative reports should address patient status and nerve stimulation characteristics at the close of surgery. The American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) recommends treating any complications that emerge with immediate referral to a specialist.7
The International Neural Monitoring Study Group (INMSG) and German practice guidelines currently endorse the use of IONM for all thyroid surgeries.8,9 However, most of the studies showed that when IONM was compared to the routine visual identification (VA) of RLN, there were no statistically significant differences in the overall transient or permanent RLN palsy.10–14 Recent AAO-HNS guidelines also suggest using IONM in thyroid surgery to optimize voice protection.15 However, the American Head and Neck Society encourages IONM utilization in surgeries for thyroid cancer, especially those associated with a pre-established RLN abnormality. Routine utilization of IONM remains a controversial topic among thyroid surgeons. In this chapter, some of these guidelines and controversial opinions are reviewed.
Recurrent laryngeal nerve injury may have permanent or transient effects. In the hands of experienced thyroid surgeons, <1% of such injuries should result in permanent damage. Remarkably, reported rates are as high as 10%.16–18 Given that surgeons accruing the least satisfactory results are less inclined to report RLN injuries and that reported RLN injuries typically emanate from high-volume practices, the published rates of RLN injury are very likely underestimated.19–21 Rates of complications related to thyroid surgery, including RLN injury, are generally higher in low-volume surgical settings.22–24
Revision thyroid surgery may be challenging, due to postoperative scarring. Compared with initial procedures, the risk of RLN injury increases threefold in revision surgeries.25
Reported rates of EBSLN injury (0% to 58%) vary widely due to symptom variability and difficulty in assessment.26
Since its introduction, and especially within the past decade, IONM has gained popularity.27 In recent years, roughly 65% of otolaryngologists and 53% of general surgeons use IONM for some or all of their thyroid surgeries.28,29 Of note, 50% of high-volume surgeons (performing ≥100 thyroid operations per year) use neuromonitoring, compared with 22% of low-volume surgeons.28
Right nonrecurrent inferior laryngeal nerve is more likely to be injured as it runs an aberrant course. The nonrecurrent inferior laryngeal nerve originates in the neck from right vagus nerve. Its prevalence is estimated to be about 0.5% to 1.0%.5 It is usually associated with an aberrant right subclavian artery (arteria lusoria) originating from distal aorta. IONM may be used to determine the latency of nonrecurrent inferior laryngeal nerve, reflecting the distance between point of stimulation and site of response (i.e., vocal cords). Median latency of normal RLN is 4.6 ms, compared with 2.7 ms for nonrecurrent inferior laryngeal nerve.30
The reported rate of extralaryngeal bifurcation ranges from 20% to 95%.31 Extralaryngeal branching of RLN heightens the risk of being vulnerable to injury.32 The anterior branches are usually motor nerves. In this context, false negatives may result if branches that do not supply posterior cricoarytenoid muscle are stimulated.33
Right-sided RLN branching is more likely to occur (right, 59%; left, 41%), and concomitant bilateral branching has 27% rate of occurrence.34
Laryngeal nerves are more vulnerable to injury during revisions of thyroid surgery and following central neck surgery or radiotherapy, due to associated extensive scarring. A large substernal goiter, dissection of central compartment lymph nodes, Graves’ disease, and severe thyroiditis may stretch regional nerves, creating tension and heightening risk.
Nonrecurrent inferior laryngeal nerve deviates from its usual course and is associated with higher risk of injury. Extralaryngeal branching also heightens risk, due to the complexity of dissection required, as opposed to exposing a much larger single nerve.
Traction, thermal injury, suture entrapment, and transection are the main types of trauma to laryngeal nerve during thyroid surgery. In endoscopic thyroidectomy, where sutures and clamps are not used, thermal injury and traction are largely responsible for damage to RLN.35 The energy-driven and heat-generating devices used are capable of collateral thermal damage to nearby structures, including laryngeal nerves.36 Traction injuries typically occur during specimen extraction through small endoscopic incisions.
According to the International Neural Monitoring Study Group (INMSG), nerve injury is classifiable into two types.37 Type 1, or segmental injury, may be corrected (if entrapment or compression by suture is the cause) to avoid permanent injury. Type 2, or global injury, is not localized to a given nerve segment but affects conductivity of the entire nerve and may be due to an intralaryngeal focus of injury.
Segmental nerve injury is more often seen with direct trauma, namely transection, ligation, bipolar coagulation, or clamping, whereas global injury generally results from indirect forces, such as traction applied to the trachea or adjacent tissues.38
Recurrent laryngeal nerve (RLN) origin: RLN originates from vagus nerve at right subclavian artery level on the right side and at aortic arch on the left. The right RLN curves around right subclavian artery and ascends within tracheoesophageal groove to innervate the larynx. The left RLN originates in superior mediastinum, loops around aortic arch, and travels cephalad (via thoracic outlet), runs in the tracheoesophageal groove near distal branches of inferior thyroid artery. Prior to entering the larynx, it supplies sensory branches to esophagus and trachea.
Intraoperative landmarks for identifying RLN: The four important landmarks for locating RLN during thyroid and parathyroid surgery are the following:5,31
Tracheoesophageal (TE) groove: The course of RLN in TE groove is not constant in all individuals and tends to vary more on the right side. On the left, RLN ascends in a paratracheal position in or near TE groove in 60% to 70% of cases. The right RLN takes this path only 59% of the time.39
Entry to larynx: Most surgeons would agree that this is the most reliable landmark for RLN. However, this is also the most common site of RLN injury. RLN runs posterior to thyroid gland before passing through lower fibers of inferior constrictor muscle (its laryngeal entry point). The extremely close proximity of RLN to thyroid capsule and ligament of Berry assures vulnerability.5
Inferior cornu of thyroid cartilage: This particular landmark of RLN signals its entry to larynx and is manually palpable.
Inferior thyroid artery: This is an important (albeit variable) landmark of RLN.
Common sites of RLN injury are at its intersection with inferior thyroid vessels and at the point of entry at the ligament of Berry. The ligament of Berry also closely approximates RLN as the nerve passes through the ligament (25% to 30%) or deep to it (i.e., dorsal, 75%).39
External branch of superior laryngeal nerve (EBSLN) origin: Superior laryngeal nerve originates from vagus nerve at C2 spinal level, promptly dividing (1.5 cm distal to its origin) into internal and external branches. EBSLN then descends toward larynx in a position dorsal to carotid sheath and superior thyroid artery and superficial to inferior thyroid constrictor. The superior pole of thyroid is where EBSLN may readily be explored. The laryngeal head of sternothyroid muscle serves as a landmark for locating this nerve. At the lower part of the anterolateral aspect of cricoid cartilage, the EBSLN splits into two branches that enter separately into pars obliqua and pars recta, the two heads of cricothyroid muscle. In patients with large goiters and masses involving the upper pole of thyroid, dividing the upper edge of sternothyroid muscle transversely, then applying traction (caudal and lateral) to the upper pole and dissecting the sternohyoid-laryngeal triangle bluntly improves EBSLN exposure.
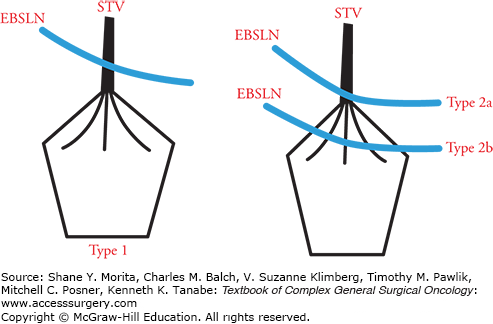
The close proximity of EBSLN and superior thyroid vessels leaves the nerve vulnerable during vascular ligation. However, relative positioning of nerve and vessels is not constant, leading some to research and classify potential variations (relative to superior thyroid vessels, inferior constrictor muscle, and superior pole of thyroid).40–42 Cernea et al40 have identified three subtypes of EBSLN in relation to upper pole of thyroid and superior thyroid vessels (Fig. 37-1).
FIGURE 37-1
Cernea et al classification of the EBSLN according to relation to the superior thyroid vessels and the upper thyroid pole. Type 1: EBSLN crosses superior thyroid vessels more than 1 cm above upper thyroid pole. Type 2a: EBSLN crosses superior thyroid vessels less than 1 cm above upper pole. Type 2b: EBSLN crosses superior thyroid vessels below upper border of the thyroid gland. EBSLN, external branch of superior laryngeal nerve; STV, superior thyroid vessels.
EBSLN may continue through cricothyroid membrane to share innervation of anterior thyroarytenoid muscle with RLN as the communicating nerve. Sanudo et al43 estimate this occurrence at 68% to 90%. Similarly, Maranillo et al44 cite an 85% occurrence rate for the communicating branch.
IONM may prove highly beneficial in identifying EBSLN, especially in patients where the nerve runs deep to inferior constrictor fascia and is difficult to spot visually.26
Endotracheal tube-based surface electrodes have become the most common monitoring technique, surpassing other modalities (i.e., glottic observation, laryngeal palpation, glottic pressure monitoring, endoscopically placed intramuscular vocal cord electrodes, intramuscular electrodes inserted through cricothyroid membrane, and postcricoid surface electrodes) in safety, simplicity, and overall benefits.8
Grounding electrodes (for stimulator probe and recording electrodes) and a connector box are needed to complete the monitoring system circuitry.
The advantages of utilizing endotracheal surface electrodes include the large areas of contact made with target muscles, summated electromyography (EMG) capability, easy setup, and noninvasiveness. Cricothyroid muscle twitch is monitored in response to EBSLN stimulation, as opposed to analyzing EMG waveform reception.26
The use of needle electrodes does entail some complications, such as hematoma, infection, or even retained broken needle tips.
Recently, some authors suggested potential benefits of continuous IONM (C-IONM), which entails continuous electrical vagal stimulation. A segment (<1.0 cm) of vagus nerve is dissected to facilitate application of the continuous monitoring electrode.38 C-IONM theoretically could allow an immediate detection of adverse EMG events; hence, an instigating surgical offense may be aborted to avoid vocal cord paralysis.
For monitoring purposes, many surgeons consider equipment offering visual waveform data to be superior to the audio systems that only sound alarms when detecting abnormal signals. Visual waveform equipment displays quantitative amplitude, latency, and threshold data, which (unlike audio systems) help differentiate true signals from artifacts. Systems combining both audio alerts and visual waveform displays are available as well, adding an alarm to trigger scrutiny of the accompanying visual monitor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






