© Springer Science+Business Media New York 2016
Marina Kurian, Bruce M. Wolfe and Sayeed Ikramuddin (eds.)Metabolic Syndrome and Diabetes10.1007/978-1-4939-3220-7_1919. Intragastric Balloon
(1)
Universidad Pedro Henriquez Urena, Santo Domingo, Dominican Republic
Keywords
ObesityDiabetesIntragastric balloonWeight loss interventionEndoscopic bariatric procedureEndoluminal therapiesObesity is a serious, complex disease and both its treatment and management have remained a global medical challenge. It has been proven that obesity reduces the quality of life as well as life expectancy in patients and is associated with numerous comorbidities including but not limited too diabetes mellitus, obstructive sleep apnea, hypertension, lipid disorders, hepatic steatosis, ischemic heart disease, certain cancers amongst other illnesses.
Body Mass Index (BMI) is used to classify weight, with overweight BMI 25.0–29.9 kg/m2, obesity as class I BMI 30–34.9 kg/m2, obesity class II BMI 35–39.9 kg/m2, and obesity class III BMI > 40 kg/m2. Based on data obtained from the National Health and Nutrition Examination Survey 2007–2008, 68 % of adults over the age of 20 years in the USA are overweight or obese; 33.8 % are Class I or above. 14.3 % having Class II and 5.7 % have Class III obesity (1), (2). The World Health Organization projects that by 2015, approximately 2.3 billion adults will be overweight and >700 million will be obese (3).
To date surgical therapies has been the only effective sustained option to treat obesity, efficiently reducing not only body weight and weight related comorbidities in up to 80 % but also aiding in the maintenance of weight loss (4), (5).
The World Health Organization has recommended a decrease of 5–15 % of total body weight maintained throughout time to reduce the incidence of morbidities related to obesity (6)–(8). The National Institute of Health recommends lifestyle changes remain the first line of therapy for successful weight loss, these include healthy eating habits, increased physical activity with exercise, as well as psychotherapeutic support (9) possibly supplemented by medication as second line therapy (10), (11). Unfortunately multiple studies report that these noninvasive therapeutic approaches have limited sustainability for the vast majority (>90 %) of those attempting these lifestyle modifications, resulting in frustration at not losing the amount of weight desired and after a variable amount of time an increase back to their original weight.
So to those many patients who have failed these conservative therapeutic methods but who are not yet ready or may not even qualify for a more aggressive surgical approach, what can we offer them? Ideally a less invasive option, with a lower risk profile and reduced costs, we offer them an endoscopic option.
Endoluminal therapies have the potential to extend treatment options to those patients with multiple comorbidities, older age, and those who do not qualify for surgical interventions such as type I obesity (BMI 30–35 kg/m2) or even overweight patients (BMI 25–29.9). They can also supply a possibly reversible treatment modality avoiding committing to permanent surgical modifications of the gastrointestinal (GI) tract; this can be particularly attractive to a certain patient population (12).
One of the endoluminal/endoscopic bariatric therapeutic options is space occupying devices, which take form of a temporarily placed prosthetic balloon. The intragastric balloon is one of the earliest devices placed endoscopic as a weight loss intervention and continues to date as the most common endoscopic bariatric procedure performed worldwide, because of this it is the most widely studied of the minimally invasive endoscopic therapies for obesity.
The effect is intake restriction by mechanical space occupying artifact; this enhances satiety and instigates weight loss (13). The intragastric balloons are placed perorally as an outpatient procedure, with endoscopic assistance both for insertion and removal.
So how do these intragastric balloons actually work? Conceptually, they function on a mechanical basis, although other mechanisms of action may include delayed gastric emptying, hormonal(14). Other non-balloon space-occupying technologies being developed include polymer pills that expand and later degrade in the stomach thereby eliminating the need for endoscopic insertion and removal (15).
In 2011, an ASMBS Task Force determined that the primary goal of endoluminal/endoscopic bariatric therapies (EBT) was to induce enough weight loss to decrease obesity related metabolic comorbidities and improve quality of life. Endoluminal therapies, such as the intragastric balloon, have many potential applications as primary, early intervention/preemptive therapy, bridge therapy, adjunctive, or revisional bariatric procedures (16).
Although various novel endoscopic interventions and endoscopically placed devices have been described over the past decade, and several such transoral endoluminal procedures are currently under investigation in the USA, none of them have been formally approved for use in the USA (11). Until formally approved by appropriate regulatory authorities, their use remains limited to clinical trials (16).
19.1 History of the Intragastric Balloon
Nieben proposed in 1982 the use of a gastric balloon for control of obesity after having observed that a gastric bezoar (space occupying intragastric mass) had been well tolerated for a long period of time and produced significant weight loss (17). And so the first artificial space occupying intragastric balloon for weight loss was used.
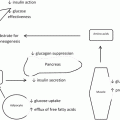
Theoretically the intragastric balloon affects both stretch receptors and gastric capacity, increasing satiety while decreasing residual volume available for food; therefore, this could be considered a non-surgical restrictive weight loss procedure.
In the early 1980s, several intragastric air-filled pouches with filling volumes of 20–500 ml were proposed. Amongst them the Garren–Edwards balloon required 220 ml, the Ballobes intragastric balloon inflated to 475 ml of room air and the Taylor intragastric balloon was smooth silicone pear-shaped filled with 550 ml of saline, were among those tried. Being that the cylinder shape devices were air filled and light, no weight effect was produced onto the stomach walls.
In 1985, the first widely used intragastric balloon, the Garren–Edwards Gastric Bubble (GEGB), was approved for use in the USA, as an adjunctive modality to a multifaceted approach to obesity. The GEGB was a polyurethane cylindrical device with a self-sealing valve through which a removable air-insufflation catheter was inserted, filled to 220 ml (unclear as to why this volume was chosen) then left to float freely in the stomach and was removed endoscopically after being punctured with a forceps.
In the late 1980s, several sham-controlled studies were published showing that diet and behavior modification were equally as efficacious as the GEGB in producing weight loss (18)–(20). Additionally these initial intragastric balloons had an elevated complications rate from: gastric erosion, 26 %; gastric ulcer, 14 %; Mallory–Weiss tears, 11 %; complete deflations, migrations, and intestinal obstructions. So after an initial enraptured period, a critical phase followed due to the failure and/or high complication rate of the Garren–Edwards, Ballobes, Taylor and Wilson–Cook balloons (21)–(25). More than 25,000 GEGB were placed before its withdrawal from the market. In addition, several polyurethane balloons (kept in place by a nasogastric catheter taped to the nose) were tried, with similar results (43), (44). None of these renditions were widely used in clinical practice. Other balloons used in the 1980s are now obsolete and had little controlled data, including devices produced by Wilson-Cook (Winston Salem, NC), Tremco (Cleveland, OH), and Dow-Corning.
In 1987, 75 international experts met and participated in a workshop on “Obesity and the Gastric Balloon” decided against a recommendation for removal of existing gastric balloons from the market but urged that their use be discouraged outside of controlled investigational trials. They also formulated and defined fundamental requirements for optimal, effective and safe intragastric balloon design. Years of research resulted in the development of a balloon that fulfilled the specified requirements: (1) the balloon should be smooth, seamless, and constructed of long-lasting material, with a low ulcerogenic and obstructive potential; (2) incorporation of a radiopaque marker to allow appropriate follow-up in case of deflation; and (3) the ability to adjust the balloon to a variety of sizes and to fill it with fluid instead of air (26).
Today intragastric balloons are no longer available in the USA; a few newer intragastric balloons are under investigation for FDA approval.
19.1.1 Types of Balloons
19.1.1.1 Bioenterics Intragastric Balloon/Orbera
The Silicone Intragastric Balloon (SIB) was developed by Fred C. Gau in conjunction with INAMED Development Company (IDC) in 1986. In January 1996, the SIB IDE was transferred from IDC to BioEnterics Corporation (BEC) and the SIB was renamed the BioEnterics Intragastric Balloon (BIB). The BioEnterics Intragastric Balloon (BioEnterics Corp., Carpinteria, Calif.) meets the 1987 requirements (40).
The currently used Orbera™ intragastric balloon (Allergan Inc., Irvine, CA, USA) is a spherical, large capacity silicone polymer device. The deflated balloon comes preloaded on a catheter, which is blindly passed transorally into the esophagus then an endoscope is passed along side it to ensure accurate placement of the balloon in the fundus. Under direct endoscopic visualization, the device is inflated through the external port of the catheter with 400–700 ml saline and 10 ml methylene blue dye solution. In case of balloon rupture the dye is systemically absorbed and imparts a characteristic blue color to urine alerting the patient to contact the physician for urgent endoscopic removal of the device. The balloon is currently deployed for a maximum duration of up to 6 months, after this time there is a higher risk of spontaneous balloon deflation. When required the device can be safely deflated and extracted endoscopically using a snare or basket.
Other available IGBs include the Heliosphere (IHB) (Helioscopie, Vienne, France), Silimed (Silimed, Rio de Janeiro, Brazil), and Semi-stationary Antral Balloon (JP Industria Farmaceutica, Ribeirao Preto, Brazil).
19.1.1.2 Heliosphere
The placement of the Intragastric Heliosphere Bag (Helioscopie, Vienne, France) an intragastric device insufflated with air instead of fluid, introduced in 2004. The balloon is slowly inflated with 840–960 cm3 of air, which gives the inflated final volume of 650–700 cm3, as the air is compressed (27). Data regarding the efficacy and safety of IHB are limited.
19.1.1.3 Silimed
The Silimed Gastric Balloon (Silimed Silicone Instrumental Medical-Surgical Hospital Ltd., Rio de Janeiro, Brazil) is consists of a smooth and transparent silicone shell that acquires a round format when filled with saline solution, it is supplied empty delicately rolled up inside a thin silicone sheath. During placement, the extremity of SGB’s sheath, not of the shell, is carefully anchored to the endoscope extremity using a polypectomy snare then it is smoothly inserted into the stomach by traction under direct visual examination. When the device is near the pyloru it is released by the polypectomy snare and positioned in the gastric fundus by “J” maneuver, followed by SGB traction by the introduction catheter. After adequate placement in the gastric fundus and under continuous direct endoscopic visualization the SGB is filled with saline solution through a tube with a polytetrafluorethylene needle at its extremity, which is connected to a self-sealing valve attached to the device shell. The volumes of saline solution (mean of 632 ml), Iopamiron® contrast (20 ml), and 2 % methylene blue (10 ml) were fixed with the approximate final proportion of 65:2:1 (28).
The SGB removal procedure consists of first positioning a lubricated double silicon overtube in the patient’s esophagus. Then under direct endoscopic observation, a hole is made into each SGB by a specially developed catheter containing a needle (Scorpion) to empty via the catheter the balloon. Then the emptied SGB is captured by a polypectomy snare and pulled until part of the SGB was held in the overtube. For the very flat balloons a double-hook endoscopic forceps can be used to bring the balloon partially inside the esophagus, the grasping it with the polypectomy snare, allowing the simultaneous removal of the balloon along with the whole endoscopic apparatus. Both the procedures are performed under usual sedation of diagnostic endoscopy (28).
Silimed Gastric Balloon has a radiopaque mark around the valve, using Iopamiron® in the filling solution of the device contributes to obtain more clearly defined images of the balloon to verify the correct placement, whenever necessary (28).
19.1.1.4 Reshape
From ReShape Medical (San Clemente, CA), the previously known ReShape Intragastric Balloon, now newly branded as the ReShape Duo is a unique dual-balloon filled with an evenly distributed 900 ml of saline. The proximal balloon sits high in the fundus, possibly contribute to increased satiety, the design conforms easily to the curvature of the stomach for stability and provides significant protection. The dual balloon design potentially reduces the undesirable risks of migration, obstruction, and perforation. In the case that one of the balloons deflates, because it is a dual-balloon device, the second balloon will maintain the ReShape Duo within the stomach, preventing migration and possible bowel obstruction. This enables the patient enough time to return to the physician for safe device removal (29).
The ReShape Duo is endoscopically delivered over a standard guidewire and automatically inflated with 900 ml sterile saline solution with a power pump delivering 450 ml to each balloon. The device is removed endoscopically after a controlled and rapid fluid evacuation with the ReShape Removal Catheter (29).
19.1.1.5 Obalon
The most recent addition to the intragastric balloon devices, commercially launched on a limited basis in Europe beginning in July 2012, is a novel swallowable gelatin capsule which dissolves inside the GI tract, that contains the balloon folded inside, attached to a miniature catheter via which the balloon is rapidly inflated and afterwards the catheter is easily removed without need of endoscopic assistance or sedation. The Obalon is a 250 ml gas filled balloon which resides in the fundus. The high buoyancy of the device allows it to occupy an area high in the gastric space perhaps allowing lower balloon volume to stimulate weight loss Additional balloons, up to three in total, can be swallowed and inflated to increase total resident volume throughout a 3-month treatment period to further stimulate weight loss. The swallowing and inflation of the balloons averaged 5 min. Balloons were removed via endoscopy using standard tools under light, conscious sedation and averaged 10 min. Minimal symptoms are reported, the ability to gradually add balloon volume appears to improve treatment and tolerability.
At the end of the 3 months, during a short endoscopy utilizing common standard tools, all balloons are removed. Safety and efficacy data were collected and reported on the first commercial product uses at 11 centers throughout Belgium, Germany, Italy, and Spain (30).
19.1.1.6 Adjustable Intragastric Balloon
The advantages of an adjustable balloon to provide improved patient comfort and hence offer greater efficacy are being investigated. The Spatz Adjustable Balloon system (ABS) has a migration prevention function to ideally enable safe prolonged implantation. Longer implantation duration could improve efficacy and weight maintenance post-extraction.
19.1.1.7 Mechanism of Action
Placement of an intragastric balloon results in a complex interplay of neurohormonal factors and changes in gastric motility, in addition to the obvious space-occupying effect. Several studies based on animal and human data, have shown that an effect on satiety and subsequent caloric intake is only seen after distention of the intragastric balloons to at least 400 ml (39).
A study done by Bonazzi et al. aimed to analyze the influence of an intragastric balloon on gastric emptying in obese patients. Twelve patients were included in the study, with BMI mean 38.51 SD ± 4.32 kg/m2. The intragastric balloons inserted were BIB under light anesthesia, utilizing direct visualization via endoscopy they were inflated with 700 ml of saline and removed 6 months later.
The measurements obtained besides body weight for gastric emptying were T1/2 and Tlag using 13C-octanoic acid breath test. These were documented prior to balloon placement, during its permanence and 2 months after removal. Gastric emptying rates were significantly decreased in the first periods while the balloon was in the stomach, and these values returned to pre-implantation values after the IGB was removed. T1/2 was: 87 ± 32 min before BIB positioning, 181 ± 91 min after 1 month, 145 ± 99 min after 3 months, 104 ± 50 min after 6 months and 90 ± 43 min 2 months after removal. T lag was 36 ± 18 min before BIB positioning, 102 ± 82 min after 1 month, 77 ± 53 min after 3 months, 59 ± 28 min after 6 months and 40 ± 21 min. Two months after removal (31).
So it appears that intragastric balloons in obese patients seem to aide patients in following the hypo caloric diet, especially during the first 3 months when the gastric emptying is slower and the sense of repletion is higher. Unfortunately after this period, the gastric emptying starts to return to normal maybe signaling that the stomach is adapting to intragastric balloon.
Ghrelin is an important gut hormone; a study led by Martinez-Brocca measured the effect of a BioEnteric Intragastric Balloon on the level of this hormone in morbidly obese patients who were considered treatment-resistant. Twenty-one participated in this randomized, double blind, sham controlled 4 month trial. Monthly anthropometric and biochemical parameters, estimation of energy intake, and preprandial and postprandial evaluation of satiety were required. Ghrelin response after a standard mixed meal was measured prior to BIB placement and 4 weeks after the endoscopic procedure (32).
There was no significant difference in weight loss between the Group Balloon and Group Sham at any time-point of the follow-up. Patients from Group Balloon did show a temporary increased preprandial and postprandial satiety, this was noted to have been maximal at 4 weeks after the intervention. Total area under the curve, fasting and postprandial plasma ghrelin were not significantly different between groups at inclusion or 4 weeks after follow-up. Therefore no correlation was found between any of the satiety scores at any time-point with their comparable ghrelin levels. From this study we can conclude though that BIB induces a temporary sense of satiety in morbidly obese patients, this is not mediated by modification of fasting or postprandial levels of plasma ghrelin (32).
Cholecystokinin (CCK), an important regulatory hormone involved in satiety, is produced in the duodenum and is stimulated by both the presence of digestion products in the stomach, mainly fats and proteins, and also by gastric distention/stretching. It acts not only on pancreatic enzyme secretion, gallbladder contraction, and increased gastric vagal afferent activity, CCK in addition delays gastric emptying and causes pyloric constriction. It has been shown that infusion of CCK, in combination with gastric distention, significantly reduces food intake in humans, and this effect is thought to be due to a CCK-mediated delay in gastric emptying. Knowing the effects of this regulatory hormone, we can imply it plays an important role in the physiologic effect of intragastric balloon placement (33), (34).
Short-term satiety is principally affected by gastric distention and gastric volume. In both animals and humans, short-term food intake is affected by the weight and volume of food more than its energy content or caloric value (35)–(37). Rolls et al. (38) showed that by infusing high-volume, low-calorie gastric feedings there was subsequently a decrease in caloric intake of a buffet meal, this was compared to similar degree with a high-volume, high-calorie gastric feeding. This volume regulated satiety is thought to result primarily from gastric distention. Mechanical gastric balloon distention to a volume greater than 400 cm3 during meals significantly reduces oral intake, and even lesser volumes may have an effect in achieving satiety (39).
Results
In 2011, ASMBS joint Task force determined that the most commonly used end-point in bariatric studies was percentage excess weight loss (%EWL). “Excess weight” being the difference between the patient’s weight and the average weight of a standard individual with body mass index (BMI) of 25 kg/m2. The weight loss achieved after bariatric intervention is calculated as a percentage of pre-intervention excess weight, this is the %EWL (16).
BIB
Of all the available intragastric balloons, the BioEnteric Intragastric Balloon has been the most widely used since 1995 in well over 20 countries worldwide, particularly in Europe, South America, and Asia (35). Probably because of this widespread use it has the highest number of publications than any other intragastric device. The indications for BIB use may be summarized as the following: (1) preoperatory weight loss in a patient candidate to bariatric surgery with high anesthesiological risk, (2) temporary weight loss treatment in a patient with body mass index (BMI) in the range of bariatric surgery (>35) who refuse surgery or has possible low compliance to surgery or in case of very long waiting list, and (3) temporary weight loss treatment for a patient with no indications to surgery in the context of an integrate medical approach to obesity (BMI < 35).
To be able to clearly advise a patient on weather or not an intragastric balloon will be helpful in their situation, we must first evaluate all the possibilities. Are intragastric balloons better than lifestyle modifications of diet and exercise alone for weight loss?
A study by Genco et al. (41) compared in a retrospective manner 130 patients with BIB placement with a 130 patients who underwent structured diet therapy with simple behavioral modifications for 6 months. A caloric restricted diet of 1000–1200 cal/day using an approximate macronutrient distribution, comparable to the “Mediterranean diet ,” including 25 % protein (at least 60 g/day), 20–25 % lipids, and 50–55 % carbohydrates. In the BIB group, patients received just generic counseling for eating behavior. In both groups considered weight loss parameters (kilograms, percentage of excess weight loss [%EWL], body mass index [BMI], percentage of excess BMI loss [%EBL]) at 6 and 24 months from baseline and comorbidities at baseline and after 24 months.
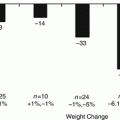
At 6 months time, BIB was removed significantly better results in terms of weight loss in kilograms 16.7 ± 4.7 vs. 6.6 ± 2.6; p < 0.01, BMI 35.4 ± 11.2 vs. 38.9 ± 12.1; p < 0.01, %EBL 38.5 ± 16.1 vs. 18.6 ± 14.3; p < 0.01, and %EWL 33.9 ± 18 vs. 24.3 ± 17.0; p < 0.01 were documented in patients treated by intragastric balloon as compared to diet-treated patients. All these parameter findings were statistically significant.
At 24 months from baseline, patients treated with intragastric balloon have tended to regain weight, whereas diet-treated patients have already regained most of lost weight. But we can state that in the short-to-medium term, BIB is significantly superior to diet in terms of weight loss.
The BIB has been compared with surgical treatment , specifically the sleeve gastrectomy, in two nonrandomized studies. At 6 months, one study showed no difference in mean weight loss, although the surgical procedure was shown to be superior at the 12-month follow-up (42). The second study of superobese patients (BMI > 50) found that sleeve gastrectomy patients lost significantly more weight at 6 months (45.5 kg vs 22.3 kg) as compared to the intragastric balloon (43).
So we now have the data to prove that intragastric balloons are not comparable to a surgical intervention for weight loss, but are statistically better at promoting weight loss when compared to diet and exercise alone.
In the largest reported retrospective study using intragastric balloon, 2515 patients were analyzed (44). The aim of the study was the evaluation of the efficacy of the BIB in a large population, specifically in terms of weight loss and its influence on comorbidities. Data were retrospectively recruited from May 2000 to September 2004, 2515 patients from the database of the Italian Collaborative Study Group for Lap-Band and BIB (GILB). Patients were discharged with diet counseling (~1000 kcal) and medical therapy for the post procedure symptoms. The BIB was removed after 6 months. Endoscopic positioning and removal were both performed under conscious or unconscious sedation. Technical success was achieved in 99 % of cases, and the authors reported five cases of gastric perforation (0.19 %), of which two were fatal, it was noted that previous gastric surgery is a contraindication to BIB placement.
Preoperative comorbidities were diagnosed 56.4 % of patients, 44.3 % of these comorbidities resolved, 44.8 % improved requiring less pharmacological dosage or shift to other therapies, and 10.9 % were unchanged. After 6 months %EWL was 33.9 ± 18.7 and BMI loss was 4.9 ± 12.7 kg/m2, this along with the concomitant improvement in hypertension and diabetes values achieved significant correction in blood pressure and glycemic control.
In Brazil, Sallet et al. (45) conducted a study from November 2000 to February 2004, where 483 overweight and obese patients were treated with the BIB®. Of these 483 patients only 323 completed a 6-month follow-up, and 85 of them completed a 1-year follow-up. A multidisciplinary program involving clinical, psychiatric, physical training, and dietary approaches was part of the required guidelines for every patient.
At the 6-month follow-up subjects measurements were compared to their baseline values, and statistically significant reductions were observed in weight (15.2 ± 10.5 kg), percent excess weight loss (48.3 ± 28.1), and BMI (−5.3 ± 3.4 kg/m2) (p < 0.000). There results are similar to other studies of the BioEnteric Intragastric Balloon (40). At the 1-year follow-up, 85 patients had maintained more than 90 % of their BMI reduction.
In a meta-analysis done by Imaz et al. (46) a Methods Systematic literature review of Medline, Embase, and other information sources from inception to March 2006 was done to perform the evidence-based systematic review of the published literature, afterwards the quality of the selected studies was assessed, 15 articles were pooled (3608 patients). Meta-analysis of weighted mean difference was made using the inverse variance method.
The estimates for weight lost at balloon removal for BIB® were the following: 14.7 kg, 12.2 % of initial total body weight, 5.7 kg/m2 in BMI, and 32.1 % of excess weight. Efficacy at balloon removal was estimated with a meta-analysis of two randomized controlled trials (75 patients) that compared intragastric balloon versus placebo, the results indicated the balloon group lost more weight than the placebo group. These differences in weight lost were 6.7 kg, 1.5 % of initial weight, 3.2 kg/m2, and 17.6 % of excess weight.
This meta-analysis did make note of the scant data available after balloon removal.
So what happens to these post BIB patients 6 months or a year after the balloon is removed? Is this short time period sufficient to change patients’ lifestyle, modify their eating habits and exercise practices to maintain the weight reduction achieved with the BIB after its removal?
In a study published in 2005, Herve et al. (47) tried to answer just that. Hundred patients who received a BIB were included in a prospective study and followed for 1 year after BIB removal. The patients assisted to monthly post-implantation follow-up visits during which they were seen by the surgeon, dietitian, and if necessary, the psychologist.
The results upon BIB® removal were mean weight loss for the group of 12.0 kg. Mean percent excess weight loss (%EWL) was 39.8 %. A year after removal of the BIB® the documented mean weight loss was 8.6 kg and mean %EWL was 26.8 % for the group as a whole.
The results 1 year after removal of the BIB were found to be encouraging, specially considering it is a temporary non-surgical and non-pharmaceutical treatment for obesity that is totally reversible and repeatable. The authors recommend it for patients who have previously failed traditional methods of weight reduction. They do note that careful patient follow-up is of primary importance in avoiding complications and supporting efficacy of the treatment because concurrent behavior modification is essential for durable weight loss.
A second 1 year post BIB a randomized, double-blind trial of balloon or sham treatment of 3 months’ duration consisting of 43 patients. A preset weight-loss goal was set and if the patients (sham- and balloon-treated groups) achieved this weight they were given an additional 9 months of balloon treatment. The patients were continued to be followed for a second year post balloon (48).
The mean body mass index at enrollment was 43.3 kg/m2 were enrolled. Five patients did not meet the preset weight-loss goal and were considered nonresponders 11.6 %. Three patients were unable to tolerate the balloon 7.0 %, at the time of the endoscopy severe esophagitis was diagnosed.
In the intention-to-treat analysis, sham- and balloon-treated groups had overall weight loss of 20 kg (16.1 %) and 16.7 kg (13.4 %) after 6 months in the sham/balloon and in the balloon/balloon treated groups which was not shown to be statistically significant. After 1-year of balloon treatment, a mean weight loss of 21.3 kg (17.1 %) was achieved in all patients, 12.6 kg (9.9 %) was maintained at the end of the second balloon-free year. Forty-seven percent of patients sustained a greater than 10 % weight loss, with considerably reduced comorbidity. In those 33 patients who completed the study per protocol, the weight loss at 1 year was of 25.6 kg (EBW 20.5 %) and 14.6 kg (EBW 11.4 %) after 2 years; 55 % maintained a weight loss of greater than 10 %.
In this study the conclusion was that for patients with treatment-resistant obesity , the intragastric balloon appeared to be safe, an independent benefit of balloon treatment beyond diet, exercise, and behavioral therapy with balloon treatment for 1 year resulted in substantial weight loss, the greater part of which was maintained during the balloon-free second year.
More recently, Angrisani et al. (49) observed the almost total regain of excess weight 1 year after BIB removal in 82 patients who had refused any other kind of treatment—surgical, pharmacological, or dietetic. On the other hand, Sallet et al. (45) observed 90 % weight loss maintenance in a subset of 85 (from the total of 323) patients at 1-year follow-up. Weight loss was noted to be significantly higher in BIB-treated patients both at 6 and 18 months follow-up. Additionally, the dropout rate was significantly lower in BIB-treated patients (1 % vs. 18 %, p < 0.001).
Seeing that the data is unclear at 1 year post BIB removal and stabilization of weight is uncertain, Kotzampassi et al. (50) published a study on 500 enrolled patients, who were followed post 6 months of BIB® induced weight reduction for up to 5 years. All patients were contacted for follow-up at 6, 12, and 24 months post-removal and then yearly thereafter. Twenty-sex patients had to be excluded because of treatment protocol interruption, thus remaining 474, of these at the time of BIB® removal 79 were excluded because of %EWL less than 20 %; thus remaining 395 patients had weight loss of 23.91 ± 9.08 kg, BMI reduction of 8.34 ± 3.14 kg/m2, and percent EWL of 42.34 ± 19.07. At 6 months and 12 months, 387 (98 %) and 352 (89 %) presented with percent EWL of 42.73 ± 18.87 and 27.71 ± 13.40, respectively. At 12 and 24 months, 187 (53 %) and 96 (27 %) of 352 continued to have percent EWL of >20. Finally, 195 of 474 completed the 60-month follow-up 23 % retained the percent EWL at >20. It was observed that those who in general lost 80 % of the total weight during the first 3 months of treatment succeeded in maintaining a percent EWL of >20 long term after. Speaking in percentages of EWL > 20, we can state from this publication that from the total of 500 obese subjects, EWL > 20 % was achieved in 83 % at the time of removal, in 53 % at the time of the 12-month follow-up, in 27 % at the time of the 24-month follow-up, and in 23 % at the time of the 60-month follow-up.
In search of improving the weight loss outcomes of the patients undergoing BIB® placement, various studies have introduced the notion of sequential IGB placements.
In 2010, a study by Dumonceau et al. (51) aimed at assessing the potential benefits of repeating IGB therapy , with a prospective non-randomized multicenter trial. Hundred and eighteen consecutive patients with a BMI 34 kg/m2 were included. Nineteen patients (16 %) underwent repeat IGB placement as requested by them, 8 to prolong the first treatment and 11 after a IGB free trial.
Higher weight loss 3 months after first IB insertion independently predicted repeat therapy (p = 0.008). Median weight loss in subjects who had repeat therapy was lower with second vs. first IGB 9.0 kg vs. 14.6 kg; 30.4 % vs. 49.3 % excess weight loss; this achieved statistical significance p = 0.003. Compared to subjects with single treatment, those with repeat treatment had greater weight loss at first IGB extraction 14.6 kg vs. 11.0 kg; 49.3 % vs 30.7 % EWL and 1 year later 12.0 kg vs 6.0 kg but the difference became less than 2 kg starting at 3 years.
At final follow-up at 4.9 years approximately, the whole subject population had lost a median of 2.0 kg or 6.2 % EW and identical proportions of subjects with single/repeat treatment had ≥10 % baseline weight loss (26 %) or bariatric surgery (32 %) which was delayed in subjects with repeat vs those with single IB therapy.
In Spain, Lopez-Nava et al. [52] evaluated a population of 714 consecutively placed BIB® which were removed after 6 months, between June 1, 2005 and May 31, 2007. These patients were discharged post BIB® with drug therapy and 1000 kcal diet. Of the initial patient population 112 patients underwent a second consecutive balloon positioning, a month after the removal of the first BIB during which they received medical therapy, the second BIB® was also removed at 6 months. Consequently patients were followed up in a weekly basis. After 6 months of BIB mean %EWL was 41.6 ± 21.8, mean BMI loss was 6.5 ± 12.7. After the second balloon removal, mean BMI was 30.3 ± 7.2, mean %EWL was 31.5 ± 23.2; mean BMI loss was 2.5 ± 18.2. After 24 months of follow-up, 22 (%) patients regained the pre-BIB weight, 61 (%) regained the 45–50 % of their pre-BIB weight, and 45 remain at the weight level after BIB removal ±2 kg. Considering the current experience, the support for this sequential approach should be in patients who require a continuous weight loss, even if not significant, to avoid the patients regain weight while waiting for definitive bariatric surgery.
The consideration in obtaining satisfactory basic results in terms of resolution of comorbidities is relevant, taking into account that the risk of death from cardiovascular disease, cancer, diabetes and other diseases increases throughout the range of moderate and severe overweight to obesity.
In this Spanish series (52), the improvement or resolution of preoperative comorbidities was obtained in 140/162 (86.4 %) patients. Many prior studies have confirmed the importance of these results demonstrating the benefit of 10 kg weight loss in terms of comorbidities (diabetes, blood pressure, lipids, etc.) and the related mortality (53)–(55).
The conclusion of these authors is that a second balloon can be positioned without difficulties, achieving good results after 12 months of treatment (52).
Both prior studies have shown that although patients who underwent a second balloon insertion had greater initial weight loss, there was no statistically significant difference in %EWL at 3-year follow-up [51, 52]. Furthermore, the placement of a second balloon was linked with a trend towards greater procedure- and device-related complications (52).
A prospective study by Forlano et al. (56) analyzed the metabolic benefits of intragastric balloon placement in 130 patients with mean BMI of 43.1 kg/m2 who were maintained on a 1000–1200-kcal diet for 6 months after balloon placement. Hepatic steatosis was followed by ultrasound, and the frequency of sonographically detected advanced hepatic steatosis declined from 52 % at baseline to 4 % at the end of the 6 months. Comparable improvements were noted a well in blood glucose and triglyceride levels, these corrections indicators of medical disease highlights the multifaceted role of intragastric balloon in patients with “metabolic syndrome.”
A prospective study from November 2003 to April 2006 in the high risk superobese population examining the role of IGB as a bridge therapy prior to bariatric surgery (57). The BioEnterics intragastric balloon (BIB) was endoscopically placed in 26 high risk superobese patients preoperatively to induce weight loss to reduce the risk of surgery associated with morbid obesity. These patients had a mean body mass index of 65.3 ± 9.8 kg/m2 and severe comorbidities. After 6 months the BIB® was endoscopically removed. The mean weight loss was 28.5 ± 19.6 kg, and clinical reevaluation revealed significant improvement in patient comorbidity status permitting bariatric surgery and reducing the perioperative morbidity and mortality rates associated with the superobese during bariatric surgical procedures. Post BIB® 20 patients underwent a primary bariatric surgical procedure the day after BIB removal; 2 patients were rejected for surgery because of inadequate weight loss. This study proves that BIB placement can be considered an effective first-stage treatment of high-risk superobese patients in need of surgical intervention.
These same findings were also corroborated by Zerrweck et al. (58) with a case control study between 2004 and 2009, where the records of 60 consecutive super-superobese patients (BMI 66.6 ± 3.4 kg/m2), 23 cases with preoperative BIB® and 37 controls with no Balloon. The end point of significant adverse events was defined as the presence of at least one of the following conditions: conversion to open laparotomy, intensive care unit stay for more than 2 days, and overall hospital stay superior to 2 weeks. In the 23 cases IGB group, the intragastric balloon was maintained during 155 ± 62 days and induced a loss of 5.5 ± 1.3 kg/m2. This weight loss manifested with clinical changes documented at the time of LGBP, and was associated with a decrease in systolic blood pressure and gamma-glutamyl transpeptidase level (p < 0.05 vs. baseline). Operative time was lower in the IGB group 146 ± 47 min vs. 201 ± 81 min in controls; p < 0.01 achieving statistical significance. Significant adverse events were also found to occur less frequently after LGBP in the BIB® group (2 vs. 13 in controls; p < 0.05).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree