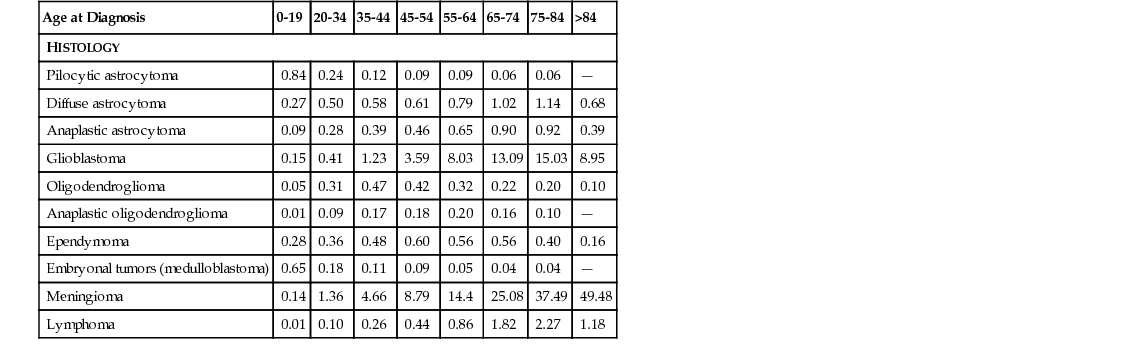
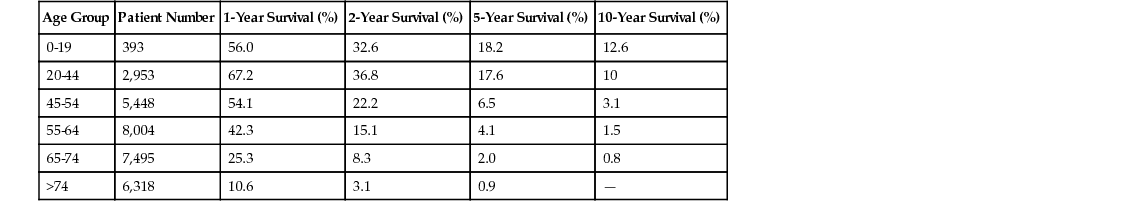
Caroline Happold, Michael Weller Intracranial tumors, as most cancers, show an increasing incidence with advancing age, with age-adjusted incidence rates for the most frequent primary brain tumors, glioblastoma and meningioma, peaking in the population aged 65 years and older, according to the most recent data from the Central Brain Tumor Registry of the United States (CBTRUS) statistical report (Table 66-1).1 In our continuously aging population, brain tumors of older adult patients have therefore become a topic of great relevance over recent decades. Overall, the incidence of specific tumor histologies, the survival prognosis, and mortality differ from those of the younger patient populations. A reduced tolerance to therapy, restricted use of therapies, and diversities in tumor biology have been discussed as possible explanations for the shorter survival of older patients with aggressive brain tumors. However, because most clinical studies in the field of brain cancer excluded older people, few data were relevant to treating these patients and therapeutic recommendations remained controversial. Some relevant information is now available from recent prospective randomized trials that studied older patients with malignant brain tumors. The leading symptom of a progressive intracranial mass is mainly a neurologic deficit in the corresponding localization, irrespective of the histologic subtype of the brain tumor. In general, primary brain tumors occur mainly in the supratentorial region: for gliomas, this comprises the hemispheres, mainly the frontal lobe (≈1/4), the temporal lobe (≈1/5) and the parietal lobe (≈1/10).1 Therefore, leading symptoms of tumor growth can include personality change and mood disorders (frontal cortex), lateralized sensory or motor symptoms (parietal and motor cortex), epileptic seizures (temporal lobes), and aphasia (mainly left-sided Broca or Wernicke region). Meningiomas, arising from the arachnoid cap cells, usually do not invade the brain but compress the cerebral cortex and can trigger the same symptoms. Neoplasias of the posterior fossa are less frequent; present with gait instability, ataxia, and diplopia; and are most likely to be of metastatic origin in older adults. More general signs of a space-occupying lesion are headaches, nausea, and morning vomiting and dizziness, all related to an increase in intracranial pressure as a result of the expanding nature of the brain tumor, as well as, especially in cases of malignant tumors and non–central nervous system (CNS) neoplasias such as brain metastases or CNS lymphomas, to the surrounding edema. Symptoms mostly develop progressively over time, especially in more slowly growing brain tumors, such as meningiomas, where manifestation of a symptom can take months or years. Some benign brain tumors are only discovered incidentally in the context of cerebral imaging for unrelated reasons. On the other hand, aggressively invading brain tumors, as glioblastomas, usually present with subacute neurologic symptoms that develop over days or a few weeks, but they can also manifest with an acute incident, such as a focal or generalized epileptic seizure. None of the presentations differs significantly in older patients when compared to younger patients, as the local distribution remains stable in all age groups. Yet, especially unspecific symptoms such as apathy or mild cognitive impairment tend to be neglected for longer time periods in older patients, as they are often misjudged as age-related dementing processes or as depression. Moreover, in patients with preexisting brain atrophy, increased intracranial pressure can manifest later than usual in the course of the disease, as the loss of parenchymal volume allows for a larger expansion of the tumor mass without occurrence of early symptoms. Acute manifestations, such as hemiparesis or epileptic seizure, are often misinterpreted as ischemic incidents initially, which strengthens the importance of additional imaging diagnostics beyond clinical assessments. The best type of imaging for diagnosing most types of brain tumors is magnetic resonance imaging (MRI), ideally contrast-enhanced MRI, which has proven to be the most sensitive imaging detection technique for brain lesions in general. Although there is no specific diagnostic imaging marker to differentiate tumor entities definitely, there are certain typical features characterizing different tumor types. Meningiomas arise from arachnoid cap cells and therefore grow extraaxially, attached to the dura mater. They are usually highly contrast enhancing and well demarcated. In contrast to these mostly benign tumors, gliomas grow diffusely, infiltrating into the brain parenchyma, usually enhancing more with higher degree of malignancy. Whereas low-grade gliomas usually show no contrast enhancement and manifest as hypointense lesions, the majority of anaplastic gliomas and glioblastomas strongly enhance gadolinium as a correlate of blood-brain barrier disruption. Glioblastomas typically present with additional central necrosis. Yet, these features can be absent in some scans, and the features of CNS lymphomas, brain metastases or even abscesses can resemble those of high grade gliomas. Eventually, no final diagnosis can be made based solely on imaging criteria, and tissue sampling via biopsy or resection is mandatory to evaluate the optimal therapeutic approach. Computed tomography (CT) scans are often used in urgent situations, when rapid imaging is required. They allow for a good identification of meningiomas attached to the dural base of the skull, where bone infiltration can occur, and can be helpful to assess calcifications in oligodendrogliomas or bleedings in metastases. Still, the poorer imaging quality in comparison to MRI and the irradiation exposure have made the CT scan a second-line choice. However, especially in patients with cardiac pacemakers or metal residua in the body, who cannot undergo magnetic scans, CT scans remain an option. The analysis of cerebrospinal fluid (CSF) can be indicated in patients with suspected brain tumors when the detection of floating malignant cells revealed by a cytologic examination might affect the therapeutic approach, for example, in CNS lymphomas or with meningeal spread of metastatic disease (neoplastic meningitis). This examination is not required for the diagnosis of a solid tumor and should not be performed without prior cerebral imaging because of the rare risk of cerebral herniation and neurologic worsening after acute decompensation resulting from CSF drain. Cerebral metastases occur in up to 30% of adult patients with systemic tumor diseases, therefore representing an issue seen more commonly in older adults.2 The most frequent source of brain metastases is the respiratory tract, followed by breast cancer and melanoma.3–5 Of note, approximately 10% of brain metastases arise from an unknown primary source. Occurrence of brain metastases always indicates a poor prognosis, and median survival from this moment on is significantly reduced to the range of 3 to 6 months,6 especially when patients have diminished reserve, as is often the case in older adults who have undergone intensive treatment of the primary tumor. In a large database analysis of patients with brain metastases, a new prognostic index, the graded prognostic assessment (GPA), was validated. It includes patient age older than 60 years as one of four prognosis-defining components that limits the median survival time. Whereas the prognostic value of age is often diminished as more factors are taken into account, the GPA was later confirmed for specific subgroups of primary cancers, and patient age was a highly significant factor, especially in the cluster of the most frequent source of brain metastases (the lung cancer patients), for both non–small cell lung cancer and small cell lung cancer.7,8 Beyond this, therapy of metastases is similar in younger or older patients, depends strongly on the systemic situation control, and can comprise either resection (in cases of a single metastasis) or palliative whole-brain irradiation. In cases of disseminated spread of metastatic tumor cells (neoplastic meningitis), patient age was identified as therapy-independent prognostic factor, with a median overall survival of 3.2 months in patients older than 60 years compared to 6.3 months in patients younger than 60 years.9 Primary brain tumors are classified according to the World Health Organization (WHO) classification based on their histologic phenotype, including neuroepithelial-derived glial cells, meningeal cells, or even lymphatic cells, as for primary CNS lymphoma, and graded from benign (WHO I) to the more aggressive forms (WHO III, WHO IV) based on their biologic behavior.5 The two most frequent tumor entities are meningiomas, classified as mainly nonmalignant primary brain tumors and accounting for 36.1% of all primary brain tumors, and gliomas, accounting for 28% of all and 80% of malignant primary brain tumors, with the WHO grade IV glioblastoma being the most important and lethal subgroup.1 Both meningioma and glioblastoma, as well as CNS lymphoma, present with a specific peak incidence in older adults, and the age of the patient represents a therapy-independent prognostic factor, depicted for glioblastoma in Table 66-2. Gliomas are the most common malignant primary brain tumors in adults, and higher age represents an independent negative prognostic factor.1,10 Even less aggressive low-grade gliomas develop a more unfavorable course of disease in older patients, leading to recommendations of an earlier therapeutic approach compared to younger patients, including surgical debulking or radiation therapy, usually up to a total dose of 50.4 Gy. The role of temozolomide (TMZ) chemotherapy for low-grade gliomas in patients of higher age (older than 40 years) versus standard radiation therapy (28 × 1.8 Gy) was investigated in the EORTC 22033-26033 study; data analysis is currently ongoing. Even so, most glial primary brain tumors in older people are the high-grade gliomas, and approximately 50% of glioblastoma patients are older than 60 years.1 Because of the markedly poor prognosis of this tumor entity, with increasing incidence especially in the geriatric population, glioblastomas are discussed here in more detail. Glioblastoma remains a fatal disease despite therapeutic advances, and population-based studies have identified age as an important prognostic factor for survival in this tumor entity, with significantly lower median overall survival in the older adult patient population.11,12 In a landmark clinical study assessing the addition of TMZ, an alkylating chemotherapeutic agent, to the former sole standard of care, irradiation, a beneficial effect on overall survival was demonstrated in a patient cohort of 573 patients between 18 and 70 years of age, with a survival improvement from 12.1 to 14.6 months in the combination arm.13 Yet, because the subgroup of patients older than 65 years was small and no patients older than 70 years were eligible for inclusion, no recommendations for the older adult patient population could be deduced from this study, making the application of this regimen to this group debatable.14 Two large Surveillance, Epidemiology, and End Results (SEER) program database analyses from the pre-TMZ era illustrate that older patients tend to be undertreated, being offered fewer treatment options (surgery, radiotherapy, or chemotherapy), reflected in a reduced overall survival of 4 months, and that combination of surgery and radiotherapy was not the standard of care in the patient population older than 70 years.11,15 In the past decade, the first randomized studies have assessed the role of different therapeutic regimens in the older adult population with glioblastoma. Keime-Guibert and colleagues analyzed the role of an irradiation regimen of 50.4 Gy versus best supportive care alone in a cohort of 85 patients aged older than 70 years and demonstrated an increased median overall survival of 6.7 versus 3.9 months.16 In the post-TMZ era, the results of two large randomized phase III studies (the NOA-08 trial and the Nordic trial) were reported in 2012,17,18 both comparing a chemotherapy-based first-line treatment to radiotherapy specifically in the older adult patient population. The NOA-08 trial compared a single-modality dose-dense TMZ regimen (7 days on/7 days off) to the standard radiotherapy with 60 Gy total in patients older than 65 years suffering from high-grade astrocytic glioma (grade III/IV). Results demonstrated the noninferiority of the chemotherapy but at the cost of increased myelotoxicity. The Nordic trial assessed two radiotherapy regimens (standard 60 Gy vs. hypofractionated 34 Gy) and a standard 5 out of 28 days TMZ regimen in patients older than 60 years, with a relevant subgroup of patients older than 70 years. Results were comparable for either TMZ or hypofractionated radiotherapy, with a worse outcome for the standard radiotherapy arm. In summary, both trials confirmed chemotherapy as an option in older patients, and both trials endorsed a higher benefit of the TMZ therapy in patients with tumors with methylguanine DNA methyltransferase (MGMT) promoter methylation. MGMT is a DNA repair protein, and methylation of the MGMT promoter has been proposed as a predictive factor for survival in patients with glioblastomas, as assessed in the patient population from the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada (EORTC/NCIC) trial, treated with alkylating chemotherapy.19 Several later studies confirmed a positive prognostic role for MGMT promoter methylation in older people with glioblastomas,20,21 and the predictive impact of the promoter methylation was confirmed in these patients.22 Therefore, the current pattern of care has shifted from radiotherapy as the only option in older glioblastoma patients to a more biomarker-driven therapeutic approach, and MGMT-methylated patients are treated with single-modality TMZ rather than radiotherapy at diagnosis.14 Other biomarkers that play a more prominent role in the younger patient population are less relevant in the older adult population; for example, IDH1 mutations, that are prognostic in glioblastoma patients, are virtually absent in older patients.23 Other age-dependent genetic alterations potentially involved in glioblastoma survival24 have not been validated as prognostic markers.
Intracranial Tumors
Introduction
Clinical Presentation
Diagnostic
Imaging
Lumbar Puncture
Classification
Secondary Brain Tumors/Metastases
Primary Brain Tumors
Gliomas
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intracranial Tumors
66