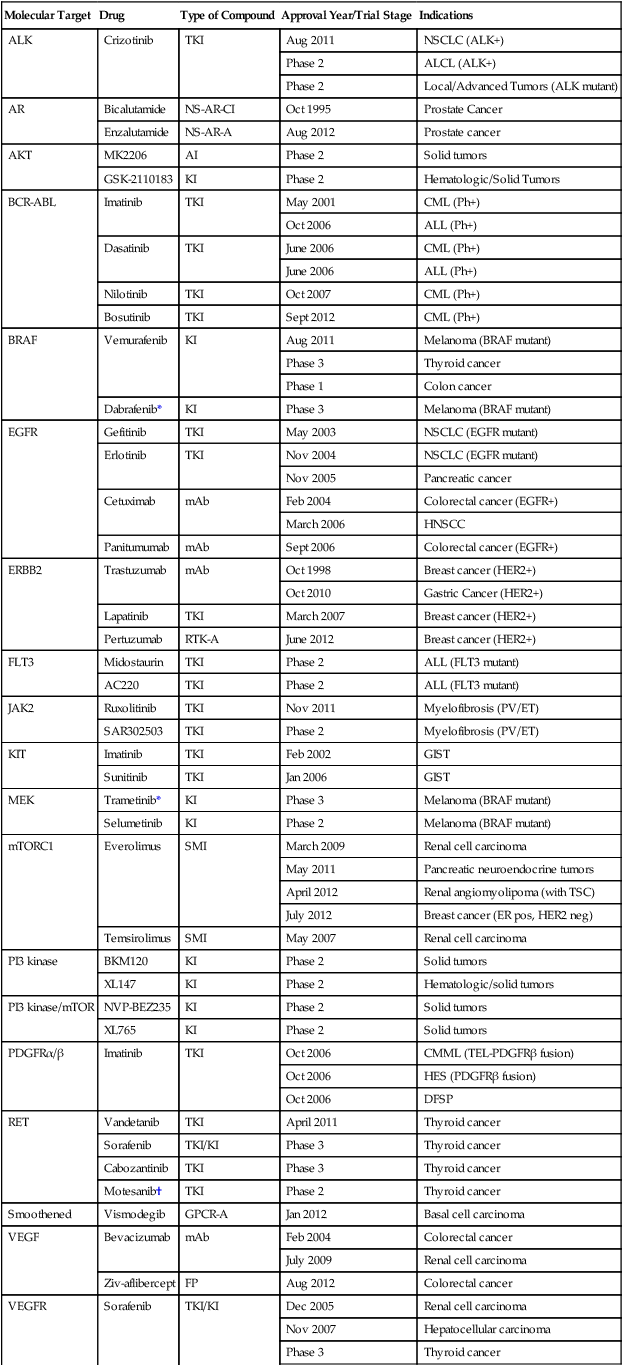
2 Aphrothiti J. Hanrahan, Gopa Iyer and David B. Solit • Ligand binding and activation of cell surface and internal receptors triggers the activation and/or suppression of signaling cascades that regulate diverse cellular processes, including cell growth, proliferation, survival, and invasion, among others. • Multiple nodes within these intracellular signaling networks are genetically and epigenetically altered in human cancers, leading to constitutive pathway activation or suppression. • A subset of cancers are dependent on genomic alterations in oncogenes or tumor suppressor genes for their growth and survival, a phenomenon known as “oncogene addiction.” • Drugs that selectively inhibit altered proteins that are critical for the maintenance of the transformed phenotype have shown unprecedented clinical activity in genetically defined subsets of cancers. • Precision medicine is the use of genetic and epigenetic information to develop treatment regimens that target the driver oncogenes and tumor suppressors responsible for tumor progression in individual patients with cancer. Potential challenges to the application of this approach include the current inability to directly inhibit some oncogenic proteins (i.e., KRAS), the development of drug resistance, technical hurdles posed by limited tissue availability for genomic studies, and intratumoral and lesion-to-lesion genomic heterogeneity. The receptor tyrosine kinases (RTKs) comprise a family of transmembrane cell surface receptors that transduce extracellular signals internally to promote growth or survival and/or regulate other cellular phenotypes.1,2 Members of this protein family share a similar modular domain structure. Growth factors bind to the extracellular ligand-binding domain of RTKs and induce dimerization of two receptor monomers, juxtaposing the intracellular tyrosine kinase domains of each monomer.3 This process results in transphosphorylation of tyrosine residues within the cytoplasmic domains of the RTK dimer. After transphosphorylation, a variety of intracellular proteins are recruited to the activated RTK through Src homology 2 (SH2) domains that recognize the phosphotyrosine plus a specific amino acid sequence motif C-terminal to the tyrosine residues.4,5 More than 117 SH2 domains have been characterized, each with unique phosphotyrosine sequence specificities.6 Each domain is part of a larger adaptor protein involved in transducing extracellular signals to activate, or in some cases suppress, specific intracellular signaling cascades. Thus the complement of signaling pathways that a given RTK regulates is dictated by the profile of phosphorylated tyrosine residues plus flanking amino acids within their intracellular domains.7,8 However, more than one adaptor protein can often recognize individual context-dependent phosphotyrosine motifs within an RTK, underscoring how this system is designed to provide both specificity and diversity of intracellular signaling. Recruitment of signaling intermediaries to the plasma membrane facilitates their interaction with membrane-bound proteins responsible for stimulating a diverse array of downstream pathways (Figs. 2-1 and 2-2). As an example, the lipid kinase phosphatidylinositol 3 kinase (PI3K), described in more detail in a later section, recognizes and binds to a pattern of phosphorylated tyrosine residues found within multiple activated RTKs through the SH2 domain located in its p85 regulatory subunit. Binding of the p85 regulatory subunit in turn results in activation of its kinase activity. Approximately 20 classes of RTKs have been defined based on growth factor specificity. This section will focus on the RTK classes for which specific cancer therapies exist or are in development. Historically, the growth factors that stimulate RTKs were discovered first, followed by the structural and functional characterization of the RTKs themselves.9,10 Epidermal growth factor (EGF) was initially purified from mouse submaxillary glands in 1962 by Stanley Cohen and was found to stimulate premature eyelid opening and incisor eruption, phenotypes that suggested a role for EGF in the regulation of cellular proliferation.11 In 1978, the epidermal growth factor receptor (EGFR) was identified as the cell surface binding site for EGF.12 During the next 2 years, tyrosine phosphorylation was identified in cells, followed by the discovery that the viral Src oncogene, which induces transformation of cells in vitro, is itself a tyrosine kinase, underscoring the potential importance of tyrosine phosphorylation for oncogenesis.13,14 Once the complete sequence of the EGFR protein was elucidated in the 1980s,15 the amino acid sequence of the receptor cytoplasmic domain was found to be similar to Src, suggesting that EGFR also possessed tyrosine phosphorylation activity. The connection between RTK activation and oncogenesis was further solidified when the amino acid sequence of EGFR was found to be homologous to the avian erythroblastosis virus erbB oncogene, which, when infected into chicken red blood cell precursors, is sufficient to induce erythroleukemia.16,17 The erbB oncogene encodes a transmembrane protein that lacks the extracellular ligand binding domain of EGFR but possesses a cytoplasmic kinase domain that, when expressed in cells, can signal in a growth factor–independent manner. Subsequent studies have since identified numerous alterations of EGFR and other RTKs within human cancers that enhance proliferation without the need for growth factor stimulation (see specific examples in the second half of each RTK subsection). The EGFR class of RTKs comprises four receptor proteins encoded by four genes (in parentheses): EGFR (ERBB1), HER2/neu (ERBB2), HER3 (ERBB3), and HER4 (ERBB4). EGFR binds to and is activated by a number of ligands, including EGF, transforming growth factor (TGF)–α, heparin-binding (HB)-EGF, amphiregulin, betacellulin, epiregulin, and epigen.18,19 Growth factor binding promotes either homodimerization or heterodimerization with other human epidermal growth factor receptor (HER) family members, followed by transphosphorylation.20 A ligand for HER2 has not yet been identified; instead, HER2 is activated through heterodimer formation with one of the other three ligand-bound receptors.21 Notably, HER2 is the preferred dimerization partner for EGFR, and EGFR-HER2 heterodimers are more stable than EGFR homodimers, remaining at the cell surface for a longer duration and undergoing endocytosis at a lower rate than EGFR homodimers.22,23 Furthermore, HER2 reduces the dissociation rate of EGF from EGFR, allowing for a more sustained period of EGF-induced signaling.18 The EGFR and HER2 components of the EGFR-HER2 heterodimer are also more likely to be recycled back to the cell surface than are EGFR homodimers, which are more readily targeted for degradation.24 Additionally, HER2-HER3 heterodimers possess the most potent mitogenic activity among the heterodimer and homodimer HER kinase combinations.25 In contrast to the other HER kinase family members, HER3 does not have intrinsic kinase activity and preferentially forms heterodimers with HER2.26 The ligands for HER3 and HER4 are the neuregulins, including heregulin. A number of tumor types frequently exhibit alterations within the EGFR family of RTKs. Sustained activation of these pathways can result in oncogene or pathway-addicted tumors, and selective HER kinase inhibitors are now a component of the standard treatment of several malignancies. Alterations within RTKs include the following: activating mutations that result in constitutive activation of the tyrosine kinase; overexpression of the receptor, often because of gene amplification; and elevated levels of RTK ligands that stimulate signaling. EGFR mutations are found in 10% to 50% of non–small cell lung cancers depending on geographic location, with kinase domain deletions (exon 19) and mutations (exon 21) comprising more than 80% of these alterations.27,28 Overexpression of wild-type EGFR as a result of gene amplification has been observed in non–small cell lung, breast, gastric, colorectal, and head and neck cancers.29,30 Up to 30% of breast cancers display overexpression of HER2, which is an unfavorable prognostic factor, and therapy for these ERBB2-amplified breast cancers is now distinct from that of breast cancers with normal HER2 expression levels.31 In glioblastoma multiforme, the extracellular domain of EGFR is frequently deleted, resulting in the expression of a truncated mutant protein (EGFRvIII).32,33 Numerous targeted agents have been developed that selectively inhibit EGFR-induced signaling (Table 2-1 and Fig. 2-2). These agents include gefitinib and erlotinib, both of which are used for the treatment of non–small cell lung cancers (NSCLCs).34 These agents are particularly effective in patients with lung cancer whose tumors harbor activating EGFR kinase mutations. Cetuximab, a chimeric monoclonal antibody that binds to the extracellular domain of EGFR and competitively inhibits ligand binding, thereby preventing receptor activation, is approved for the treatment of colorectal and head and neck cancers.37–37 Trastuzumab, a humanized antibody that binds to the extracellular domain of HER2, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of breast cancers that display HER2 overexpression. Trastuzumab has been shown to be effective both as single-agent therapy and when used in combination with chemotherapy.38,39 ERBB2 amplification strongly correlates with HER2 protein overexpression, and the presence of either marker predicts for trastuzumab response.40 Although the introduction of trastuzumab has resulted in a significant improvement in the survival of patients with HER2-overexpressing breast cancers, drug resistance remains a major clinical problem. Potential resistance mechanisms include concomitant overexpression of other HER kinase family members and/or ligands, phosphatase and tensin homolog (PTEN) loss, and the expression of a truncated HER2 protein lacking the extracellular antibody binding site.41 Additional HER2-directed agents include lapatinib, a selective tyrosine kinase inhibitor (TKI), and pertuzumab, which binds to a different HER2 epitope (the dimerization domain) than does trastuzumab, resulting in impaired dimer formation.42,43 Lapatinib is approved by the FDA for use in combination with capecitabine in patients with HER2-overexpressing advanced or metastatic breast cancer who have progressed while undergoing prior therapy with trastuzumab and certain classes of chemotherapy.42 Lapatinib also received accelerated approval for use in combination with the aromatase inhibitor letrozole.44 Consistent with its activity in patients with breast cancer, the addition of trastuzumab to chemotherapy is associated with improved overall survival compared with chemotherapy alone in patients with HER2-overexpressing esophagogastric cancer.45 Table 2-1 Select List of Current Targeted Inhibitors That Are FDA-Approved or Have Clear Activity in Clinical Trials *The combination of dabrafenib and trametinib showed promising results in a randomized clinical trial. †A phase 3 trial of motesanib plus chemotherapy in NSCLC recently failed (March 2011). ‡A phase 3 trial of sunitinib in hepatocellular carcinoma failed due to toxicity (April 2010). The insulin and insulin-like growth factor–1 (IGF1) receptor family is dysregulated in multiple malignancies.46 The insulin receptor exists as two isoforms encoded by splice variants of the same gene.47 Each isoform can dimerize with the other (forming hybrid dimers) or with itself.48 The IGF1 receptor (IGF1R) can dimerize with either of the insulin receptor isoforms or with itself, resulting in six different dimer combinations.48 The insulin receptor is stimulated by insulin or insulin-like growth factor–2 (IGF2), whereas IGF1R can be activated by either IGF1 or IGF2. Both of these latter ligands can stimulate IGF1R in an autocrine fashion or can be elaborated from distant sites.49,50 Circulating IGF binding proteins have a similar affinity for IGF1 and IGF2 as IGF1R does and therefore compete for binding to both ligands, thus titrating the amount of free ligand available for IGF1R stimulation.51 IGF binding protein proteases provide an additional mechanism for controlling ligand levels by increasing the half-life of free ligand available for receptor binding.52 After ligand binding, IGF1R dimerizes and undergoes transphosphorylation, leading to activation of downstream signaling pathways, including both the RAS-RAF-mitogen-activated protein kinase (MAPK) and the PI3K-AKT-mammalian target of rapamycin (mTOR) cascades (see individual sections later in the chapter) (Figs. 2-1 and 2-2).53 Specifically, insulin receptor substrate–1 (IRS1) binds to a phosphotyrosine motif on IGF1R via its SH2 domains and is phosphorylated by IGF1R.54 It subsequently recruits PI3K to the plasma membrane, which converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3), which results in AKT and mTOR pathway activation. Activating mutations of IGF1R do not appear to be common in human cancer. However, amplification of the IGF1R gene locus has been identified in some colon, pancreatic, and lung cancers. Sarcomas often have either increased expression of the IGF1 and IGF2 ligands or decreased insulin-like growth factor binding protein–3 expression (Ewing sarcoma), which results in increased IGF1 levels in the tumor microenvironment.55 Gastrointestinal stromal tumors (GISTs) lacking KIT and platelet-derived growth factor receptor (PDGFR) mutations also commonly harbor IGF1R amplification.56 AMG479, a monoclonal human antibody targeting IGF1R, has shown promising antitumor activity in patients with Ewing sarcoma.55 Phase 2 studies of anti-IGF1R antibodies are also ongoing in multiple sarcoma subtypes. IGF1R overexpression has also been observed in up to 44% of breast tumors and may mediate resistance to HER2-directed therapies.57 Clinical trials of IGF1R targeting antibodies in combination with hormonal and anti-HER2 therapy are ongoing. The insulin receptor family members anaplastic lymphoma kinase (ALK) and ROS1 were also recently implicated in tumorigenesis.58,59 Chromosomal translocations involving ALK and multiple 5′ fusion partners have been identified.59 In NSCLC, the EML4 gene is the preferred translocation partner, resulting in the expression of an EML4-ALK fusion protein in 2% to 5% of patients.60 Patients with NSCLC who have EML4-ALK fusions often exhibit dramatic radiographic responses to crizotinib, an inhibitor of the ALK, ROS1, and MET tyrosine kinases (Table 2-1).61 Notably, EML-ALK fusions are found in a mutually exclusive pattern with EGFR kinase domain mutations, suggesting that they have overlapping phenotypic effects. ROS1 gene rearrangements are also found in a small minority of patients with NSCLC.62,63 Crizotinib has shown promising results both in preclinical models of ROS1-fusion lung cancer and in the clinic.62,63 Platelet-derived growth factor (PDGF) is the ligand for PDGFRs, which stimulate the proliferation and migration of mesenchymal cells, such as oligodendrocyte precursors, vascular smooth muscle cells, and pericytes during embryonic development.64 PDGF signaling is also implicated in organ development, including lung and intestinal epithelial folding and glomerular capillary tuft formation. Furthermore, PDGFs promote angiogenesis, wound healing, and erythropoiesis.65 Aberrations in the PDGFR pathway result in uncontrolled proliferation and enhanced angiogenesis. Four isoforms of PDGF have been identified: PDGF-A, -B, -C, and -D.66 These isoforms are activated by proteolytic cleavage and assemble into five homodimeric or heterodimeric combinations that bind to and stimulate either PDGFR-α or PDGFR-β. PDGFR-α homodimers inhibit chemotaxis, whereas PDGFR-β homodimers and α/β heterodimers stimulate chemotaxis within fibroblasts and smooth muscle cells.67 After dimerization and transphosphorylation, PDGFRs activate signal transduction pathways through recruitment of adaptor proteins containing SH2 domains, most notably the GRB2 protein that in turn binds the guanine nucleotide exchange factor SOS, which subsequently activates RAS.68,69 Additionally, phosphorylated tyrosine residues also serve as docking sites for kinases containing SH2 domains, including PI3K, phospholipase C-gamma, and SRC, as well as the tyrosine phosphatase SH-containing phosphatase 2 (SHP2) and the signal transducer and activator of transcription (STAT) transcription factor family.69 Alterations in PDGFR signaling in cancer include excess autocrine secretion of PDGF (e.g., glioblastoma and sarcomas), gain-of-function mutations that cause constitutive tyrosine kinase activation (e.g., gastrointestinal stromal tumors),70 translocation of either the PDGF or PDGFR genes (e.g., dermatofibrosarcoma protuberans, chronic myelomonocytic leukemia, and hypereosinophilic syndrome),73–73 and PDGFR gene amplification (glioblastoma).74 PDGFR-α mutations are found in approximately 10% of KIT wild-type GISTs. The D842V mutation comprises approximately two thirds of PDGFR-α activating mutations and is resistant to inhibition by imatinib, a TKI of KIT, BCR-ABL, and PDGFRs; however, the remaining mutations are sensitive to imatinib therapy.67 Dermatofibrosarcoma protuberans is a rare, low-grade cutaneous sarcoma that harbors a chromosome 17;22 translocation that fuses portions of the COL1A1 (collagen 1A1) gene and PDGFB, resulting in overexpression of PDGF-β and subsequent stimulation of PDGFR signaling.67 Imatinib has shown significant benefit in patients with recurrent or metastatic dermatofibrosarcoma protuberans and is approved by the FDA for this indication (Table 2-1).75 Imatinib is also approved by the FDA for use in patients with KIT mutant GISTs76 and Philadelphia chromosome–positive hematologic cancers (see the SRC/BCR-ABL sections that follow). The KIT gene was first identified as the human homolog of the viral oncogene v-Kit responsible for a subtype of feline sarcoma. The KIT RTK is a member of the type III RTK family that includes PDGFR and Fms-like tyrosine kinase–3 (FLT3) (see following paragraph).77,78 Stem cell factor (SCF) is the ligand for KIT.79–82 Hot-spot mutations in exons 9 and 11 of KIT have been identified in several tumor types, including GISTs and melanomas.82–86 Patients with GISTs often have dramatic and durable responses to the kinase inhibitors imatinib and sunitinib (Table 2-1).76,87 The FLT3 receptor, a third member of the RTK class that includes PDGFR and KIT, is involved in the development of normal hematopoietic cells. It contains an extracellular region composed of five immunoglobulin domains, transmembrane and juxtamembrane domains, and two cytoplasmic tyrosine kinase domains.88 FLT3 alterations are common in hematopoietic malignancies. Specifically, internal tandem duplication within exons 14 and 15 of the FLT3 gene is found in up to one third of acute myelogenous leukemias (AMLs).89 This duplication likely results in ligand-independent activation of FLT3 and is associated with a poor prognosis in patients with AML. The clinical activity of FLT3 inhibitors has been modest to date, although responses appear to be more common in patients with FLT3/internal tandem duplication AML (Table 2-1).89 Interestingly, resistance to FLT3 inhibition in such patients is associated with selection for secondary mutations within the tyrosine kinase domain of FLT3, suggesting a central role of FLT3 in AML pathogenesis.89 Fibroblast growth factor receptors (FGFRs) comprise a family of RTKs that regulate cell proliferation, differentiation, and migration, along with selective apoptosis during embryogenesis. Germline mutations of the FGFR genes are the basis of a spectrum of skeletal developmental disorders that are thought to derive from premature differentiation and growth restriction of chondrocytes resulting from dysregulated FGFR pathway activation.90 The FGFRs are composed of an extracellular ligand-binding domain, a hydrophobic transmembrane region, and an intracellular tyrosine kinase domain.91 The extracellular domain is organized into three immunoglobulin (Ig) domains; differential splicing of the second half of the third Ig domain dictates tissue-specific expression of the receptor. Fibroblast growth factors (FGFs) bind to the extracellular domain of the FGFRs in combination with specific heparan sulfate glycosaminoglycans, inducing FGFR dimerization and transphosphorylation of intracellular tyrosine residues. Eighteen FGF ligands have been identified, and specificity for FGF receptors is based on numerous factors, including tissue-specific FGF ligand and receptor expression, the presence of cell surface molecules that facilitate the interaction between individual FGF ligands and receptors, and the differential binding capability of the ligands themselves for specific FGF receptors.92 Subsequent stimulation of the tyrosine kinase domain leads to phosphorylation and activation of multiple downstream signaling proteins in the same manner as previously described for other RTKs. Unique to the FGFR signaling complex is FGFR substrate 2 (FRS2), an adaptor protein that binds to specific phosphotyrosines on the intracellular domain of active FGFR dimers.93 FRS2 is itself phosphorylated by FGFRs and serves as a docking site for the Grb2-Sos adaptor complex that activates the RAS/RAF/MAPK pathway. Phosphorylated FRS2 also recruits GRB2-associated binding protein–1 (GAB1), which activates PI3K. Additionally, phospholipase C–γ binds to phosphorylated FGFR dimers via an SH2 domain, leading to its activation and the cleavage of PIP2 to form inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). FGFR signaling is dysregulated in cancer by multiple mechanisms, including mutational or translocation-induced activation of FGFRs, gene amplification of receptors, and autocrine or paracrine secretion of ligands by tumors. For example, activating mutations within FGFR3 result in constitutive receptor dimerization and subsequent signaling. FGFR3 mutations occur in up to 70% of nonmuscle invasive bladder cancers and in 15% of patients with advanced urothelial tumors.93 Unlike EGFR activating mutations, which predominantly affect the tyrosine kinase domain of the receptor, FGFR3 mutations are commonly located within the extracellular domain and promote ligand-independent receptor dimerization through formation of an aberrant disulfide bridge between two receptor monomers.94 Up to 15% of multiple myelomas harbor an intergenic 4;14 translocation between the FGFR3 gene and the immunoglobulin heavy chain locus, which places FGFR3 expression under the highly active heavy chain promoter.95 Approximately 10% of diffuse-type gastric cancers display FGFR2 gene amplification, and cell lines with this amplification show ligand-independent pathway activation and sensitivity to selective FGFR inhibitors.96 Autocrine and paracrine FGF ligand secretion with resultant pathway activation has been reported to occur in a subset of melanomas and prostate cancers, respectively.97,98 Multiple FGFR inhibitors are currently being tested in early-phase clinical trials, but most of these compounds are multitargeted TKIs, many of which also potently inhibit members of the vascular endothelial growth factor receptor (VEGFR) and PDGFR families. The close structural similarity between these RTKs has made development of FGFR selective inhibitors challenging, although several such drugs are now entering the clinic.93 Because FGFR3 is a cell surface receptor, it may also be susceptible to monoclonal antibody–mediated inhibition, similar to trastuzumab-mediated inhibition of HER2. To date, anti-FGFR antibodies have not entered clinical testing, although preclinical studies have shown promising antitumor effects in urothelial cancer (both FGFR3 wild-type and mutant) and t(4;14) expressing multiple myeloma cell lines.99 The RET (rearranged during transfection) protein is a membrane-bound RTK with a role in the normal development of the kidney and the enteric nervous system.100,101 RET is expressed predominantly on the surface of neural crest tissues, and glial-derived neurotrophic factors (GDNFs) serve as ligands for RET. GDNFs initially bind to GDNF receptors on the cell surface followed by binding of this complex to RET, RET dimer formation, and subsequent transphosphorylation of tyrosine residues.102 Tyrosine residues at positions 752 and 928 serve as a docking site for the STAT3 transcription factor. Other phosphorylated residues are recognized by SRC, resulting in activation of focal adhesion kinase, which promotes cell migration and metastatic spread. Additionally, the MAPK, PI3K/AKT, and phospholipase C–γ pathways can be activated by RET to promote cellular proliferation and survival.102 Germline RET mutations are the basis for the multiple endocrine neoplasia type 2 syndromes. In patients with multiple endocrine neoplasia type 2 syndrome, familial medullary thyroid carcinomas and other cancers develop.103 Sporadic medullary thyroid carcinomas are much more common, and up to 60% of such tumors harbor somatic mutations in RET that are thought to be a driver alteration in this disease. RET inhibitors have shown significant antitumor activity in patients with medullary thyroid cancer, and vandetanib, an oral inhibitor of RET, EGFR, and VEGFR, was recently approved by the FDA for the treatment of patients with advanced medullary thyroid cancer (Table 2-1).104 A randomized, placebo-controlled, phase 3 study of cabozantinib, an oral, multitargeted TKI that inhibits RET, VEGFR2, and MNNG HOS transforming gene (MET), was also recently conducted in patients with unresectable, locally advanced, or metastatic medullary thyroid carcinoma.105 This trial documented a statistically significant improvement in median progression-free survival with cabozantinib compared with placebo (11.2 months vs. 4.2 months in the placebo arm, P < .0001). Six vascular endothelial growth factor (VEGF) ligands have been identified, VEGF-A, -B, -C, and -D, along with placental growth factor 1 and 2.106 VEGF-A has four isoforms produced by alternative gene splicing, with the 165 amino acid length isoform playing a central role in tumor angiogenesis.107 Specifically, VEGF-A enhances vascular permeability and stimulates endothelial cell proliferation, resulting in new blood vessel formation. VEGFRs are receptor tyrosine kinases that possess a modular structure consisting of an extracellular domain with seven immunoglobulin-like regions, a transmembrane domain, and an intracellular tyrosine kinase domain.106 VEGF-A, VEGF-B, and placental growth factor all bind VEGFR1, but the exact role of VEGFR1 in tumor angiogenesis has yet to be fully elucidated. In some settings it may act as a decoy receptor that prevents ligand-mediated stimulation of VEGFR2.108 VEGFR2 has been implicated in the development of vasculature and is considered the primary receptor through which VEGF exerts its angiogenic effects.108 Binding of ligand to VEGFR2 results in receptor dimerization and transphosphorylation followed by activation of multiple signal transduction cascades.109 Targeted therapies that inhibit VEGF signaling include antibodies that bind circulating ligand and receptor TKIs (Table 2-1). The humanized monoclonal antibody bevacizumab binds to free VEGF, thereby preventing its association with VEGF receptors. This antibody has been approved by the FDA for use in combination with chemotherapy for patients with metastatic colorectal110 and nonsquamous NSCLCs.106,111 Bevacizumab also has activity in patients with glioblastoma112 and metastatic renal cell carcinoma, in whom it is often used in combination with interferon (IFN)-α.113 Sorafenib and sunitinib are multitargeted TKIs with nanomolar potency for VEGFR2 (Table 2-1). Sunitinib is used in the treatment of patients with metastatic renal cell carcinoma, GISTs, and pancreatic neuroendocrine tumors.114 Sorafenib has been approved for the treatment of liver and renal cell cancers.115,116 Although these agents inhibit multiple kinases, their antitumor effects have been attributed primarily to their antiangiogenic activity. More recently, the TKI pazopanib was approved for the initial treatment of metastatic renal cell carcinoma and in cytokine-pretreated patients,117 and axitinib118 was approved in the second-line setting after failure of prior systemic therapy. Despite widespread activity in preclinical models, antiangiogenic therapies have shown disappointing activity in several tumor types. A number of resistance mechanisms have been hypothesized to explain the lack of broader clinical activity, including the activation of redundant signaling pathways that promote angiogenesis, the recruitment by tumors of bone marrow–derived endothelial progenitor cells, increased pericyte density around existing blood vessels that enhances vascular growth and survival, and the ability of tumor cells to invade surrounding stroma to co-opt additional blood supply.119 A better understanding of these resistance mechanisms may lead to the development of more effective antiangiogenic therapies in the future. The hepatocyte growth factor receptor (HGFR or MET) is encoded by the MET gene.120 The MET extracellular domain consists of an α subunit connected by a disulfide bridge to a β subunit that spans the cell membrane.121 The intracellular portion of the receptor contains a juxtamembrane region that harbors a serine residue that inhibits receptor tyrosine kinase activity upon phosphorylation, as well as a tyrosine kinase domain. A tyrosine residue at position 1003, proximal to the tyrosine kinase domain, serves as an interaction site for the ubiquitin ligase CBL, which marks the receptor for endocytosis and degradation.122 Tyrosine residues C-terminal to the kinase domain represent docking sites for adaptor proteins involved in downstream signaling. Upon binding of hepatocyte growth factor (HGF) to the extracellular portion of MET, receptor dimerization occurs, followed by transphosphorylation. A number of adaptor proteins then bind to phosphorylated tyrosine residues, including GRB2 and GAB1, which promotes the activation of the MAPK and PI3K/AKT signaling pathways.123 MET can also activate RAC1/CDC42 and p21-activated kinase, both of which regulate cytoskeletal proteins and integrin expression and activation and thus cell migration.123 Dysregulation of MET signaling can occur through multiple mechanisms, including receptor overexpression, upregulation of HGF, which can activate MET in an autocrine and/or paracrine manner, and tyrosine kinase domain mutations.124 Several receptor tyrosine kinases have also been shown to activate MET, including EGFR, HER2, and IGF1R. For example, EGFR activation can stimulate MET signaling, and resistance to EGFR inhibitors in some lung cancers has been shown to stem from co-activation of MET in the setting of gene amplification.125 Germline mutations of MET are found in patients with hereditary papillary renal cell carcinomas, and MET overexpression is observed in a significant proportion of sporadic papillary cancers, as well as collecting duct carcinomas.126 Multiple other malignancies exhibit aberrations in MET signaling including lung, breast, pancreatic, colon, and gastric cancers. Amplification of MET is associated with a worse prognosis in lung and gastric cancers, and expression of MET and HGF are unfavorable prognostic biomarkers in liver, kidney, colorectal, and gastric cancers.127 Inhibitors of MET signaling have been in development for a number of years. These inhibitors include antibodies that target the extracellular domain of the receptor, antibodies that bind to and thus sequester circulating HGF, and small molecule TKIs that are both MET selective or multitargeted inhibitors.127 As previously mentioned, concurrent MET activation is a potential mechanism of resistance to EGFR inhibitor therapy in lung cancer, and clinical trials testing combinations of anti-MET antibodies or TKIs plus EGFR TKIs are ongoing. Preliminary results suggest an improvement in progression-free survival with the addition of anti-MET antibodies to an EGFR inhibitor in patients whose tumors display MET overexpression by immunohistochemistry.128 Given the limited clinical activity of selective MET inhibitors in most cancer types, predictive biomarkers of MET dependence and therefore response to MET inhibition are a focus of ongoing studies.127 G-protein coupled receptors (GPCRs) are seven transmembrane domain–containing proteins that transduce ligand-specific signals across the plasma membrane to mediate numerous physiological processes including sensory perception, immunologic responses, neurotransmission, weight regulation, and cardiovascular activity.129 GPCRs also regulate basic cellular functions including growth, motility, differentiation, and gene transcription. The GPCR family comprises more than 800 receptors, which are the targets of more than 30% of all FDA-approved drugs, though few to date have found a role as anticancer therapies.130,131 Given that GPCRs activate many of the signaling cascades that are deregulated in human cancer, it is not surprising that recent studies have implicated GPCRs in cancer initiation and progression.134–134 GPCRs can be categorized into five or six families, depending on the nomenclature used.135 Using a more recent phylogenetic classification, the five major families are represented by the acronym GRAFS: Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin.136 The rhodopsin family of receptors (also referred to as class A GPCRs) is the largest class, with more than 670 members. Crystallization of the bovine rhodopsin receptor in 2000 provided the first high-resolution insight into the structure of GPCRs.137 In general, rhodopsin family of receptors have short N-termini. Included in this family are the α group (histamine, dopamine, serotonin, adrenoceptors, muscarinic, prostanoid, and cannabinoid receptors), the β group (endothelin, gonadotropin-releasing hormone, and neuropeptide-Y), the γ group (opioid, somatostatin, and angiotensin), and the δ group (P2RYs, glycoprotein-binding FSHR/TSHR/LHCGR, PARs, and olfactory receptors).138 The 15 secretin receptors (class B) all have conserved cysteines in the first and second extracellular loop, and most have three cysteine bridges in the N-termini. This class includes the calcitonin-like, corticotrophin-releasing hormone, the glucagon-like, gastric inhibitory polypeptide, growth hormone–releasing hormone, adenylate cyclase activating polypeptide, parathyroid hormone, secretin, and the vasoactive intestinal peptide receptors.139 The adhesion family (also included in class B, according to another classification system) has distinctly long N-termini, and only 3 of 33 receptors in the family have known ligands (EGF-like module containing mucin-like receptors [EMR]-2/3/4)140. The glutamate receptor family (class C) binds ligands in their N-termini, which has a complex two-domain folded structure bridged by disulphide bonds. Common receptors in this family of 22 include the glutamate, GABABRs, calcium-sensing, the sweet and umami taste (TAS1R1-3), and the GPCR6 receptors.141,142 The Frizzled (Fz) receptors (FZD1-10 and SMO; see the last two paragraphs of this section for an in-depth discussion of signaling) bind Wnt ligands in the extracellular domain region containing nine conserved cysteine residues.143 The taste2 receptors (25 members of T2R, bitter taste) have varying sequence homologies that likely allow the sensing of thousands of distinct bitter tastes.144,145 Overall, GPCRs share the seven transmembrane domain structure but have different regions of conservation. All of the families include orphan receptors, which are related by sequence and structure, but have no identified ligand to date. Operationally, ligand binding on the GPCR extracellular surface induces a conformational change in the receptor, mainly via transmembrane helices 5 and 6, which creates a deep pocket in the intracellular face of the receptor.148–148 This cleft enables binding and activation of heterotrimeric G proteins, which consist of an inactive, guanosine diphosphate (GDP)-bound Gα subunit and a Gβγ-subunit dimer, which act as a molecular switch.149,150 Activated GPCRs promote GDP for guanosine triphosphate (GTP) exchange on the Gα nucleotide binding site.151 This GTP-bound Gα dissociates from Gβγ and the receptor, and then both activated subunits proceed to initiate signaling cascades. There are four members of the Gα family, Gαs, Gαi/o, Gαq/11, and Gα12/13, which can be further subtyped, and each can stimulate several downstream effectors. Moreover, each GPCR can couple to multiple Gα family members, thus generating a complex pattern of intracellular signaling. Classic GPCR activation of Gαs stimulates adenylyl cyclase, which generates the second messenger 3′-5′-cyclic adenosine monophosphate (cAMP).152,153 cAMP activates multiple downstream effectors including cAMP-gated ion channels, EPAC, a guanine nucleotide exchange factor for RAP1/2 (which functions in cell adhesion and junction formation), and protein kinase A (PKA).129,149,154–157 cAMP binds to PKA regulatory subunits, releasing catalytic subunits and triggering the activation of cytosolic and nuclear substrates, including the transcription factor cAMP response element binding protein (CREB), which can induce proliferation and differentiation, among other phenotypes, depending on cell origin.160–160 Gαs also activates the SRC tyrosine kinase and the guanosine triphosphatase (GTPase) activity of tubulin.149,154 GPCRs coupled with Gαi/o typically work in opposition to Gαs by inhibiting adenylyl cyclase and decreasing cAMP levels.129,161 In addition, some Gαi/o isoforms can signal to K+ and Ca+ channels, increase cyclic guanosine monophosphate (cGMP) phosphodiesterases, interact with RAP1GAP1, and cross talk to the MAPK pathway (as described later in this chapter).129 Members of the Gαq/11 family activate phospholipase C–β, which catalyzes the hydrolysis of PIP2 to yield IP3 and DAG.162 IP3 mobilizes calcium from intracellular stores, whereas DAG activates some isoforms of protein kinase C (PKC).163,164 Both Gαq/11 and Gα12/13 activate a variety of RhoGEFs (p115-RHOGEF, PDZ-RHOGEF, LARG, LBC, and AKAP-LBC) and thus regulate Rho activity (mainly RHO-A) and its contribution to actin stress fiber formation, cell shape and polarity, cell adhesion and migration, gene transcription, and cell cycle progression.165 Additionally, Gα12/13 activates the Na+/H− exchanger, inducible nitric oxide synthase, phospholipase D, E-cadherin, radixin, and protein phosphatase 5. Gβγ signaling is equally complex, as the active dimer stimulates G-protein–regulated inwardly rectifying K+ channels, adenylyl cyclase (types II, IV, and VII), PLC-β, PI3K-γ, SRC, and GPCR kinases (GRKs; see two paragraphs below), while it inhibits adenylyl cyclase (type I), some Ca+ channels, and calmodulin, stabilizes Gα in the GDP-bound, inactive state, and helps specify coupling to the proper Gα member.129,149,150,154 Gα signaling is switched off by a family of GTPase-activating proteins called regulators of G–protein signaling, or RGS proteins, which enhance the intrinsic rate of GTP hydrolysis by greater than 1000-fold.166,167 Some RGS proteins enhance signaling through RhoGEFs and act as scaffolds for other signaling cascades (e.g., tether to Raf and MEK2). The GPCR effectors PKA and PKC also contribute to receptor inhibition by phosphorylating their cognate activated GPCR and thereby uncoupling and inactivating Gαs/Gαq in a classic negative feedback loop.168,169 GPCRs can also function via G-protein–independent mechanisms by binding to the arrestin family of cytosolic adapter proteins.170,171 GPCR-coupled arrestins have pleiotropic cellular roles including (1) dampening G-protein signaling by scaffolding enzymes that degrade G-protein second messengers; (2) desensitizing receptors by binding GRK-phosphorylated GPCRs and sterically blocking access to further Gα subunits; (3) mediating GPCR trafficking and endocytosis to clathrin-coated pits; and (4) acting as a scaffold for multiple MAPK cascades. For example, MEK1 is engaged by a tethered complex of RAF-1, ERK2, and β-arrestin to facilitate mitogenic signaling.172,173 GPCRs are well-established drug targets for antihistamine, antacid, cardiovascular, and antipsychotic drugs, pain suppressants, and antihypertension therapies.130,131 Although less well appreciated as drug targets in cancer, increasing evidence indicates that dysregulated GPCR signaling contributes to cancer initiation and progression. For example, in 1986, the wild-type MAS1 gene, which encodes the MAS GPCR, was reported to induce transformation by coupling to the small G-protein RAC.174,175 Thus although mutational activation of GPCRs and G proteins may be infrequent events in human tumors, overexpression of these receptors/adapters, loss of negative regulators, or excessive ligand in the tumor microenvironment may contribute to the deregulated proliferation, angiogenesis, migration, and inflammation that is characteristic of many cancers. Aberrant signaling by thrombin, chemokines, lysophosphatidic acid (LPA), gastrin-releasing peptide (GRP), endothelin 1, and sphingosine-1-phosphate (SIP), along with prostaglandin E2, bradykinin, angiotensin II, interleukin-8 (IL-8), estrogen, orexin, and the Hedgehog (Hh) and Wnt ligands through their associated GPCRs, have been implicated in the development of a number of cancers.132,166,167 Numerous inhibitors of GPCR signaling are also being studied in the clinic, including ZD4054, ABT-627 and Bosentan (target: endothelin A/B receptors), BKT-140 (target: CXCR4 receptor), and CXCR2 ligands (target: CXCR2 receptor).133 Detailed in the following four paragraphs are a few specific examples of GPCRs that have been shown to play a role in cancer initiation and/or progression. A connection between GPCRs and EGFR signaling has been established in both normal cell physiology and in colon, lung, breast, ovarian, prostate, and head and neck cancer development.176–179 Ligand-bound GPCRs activate SRC, PKC, Ca+ channels, and PKA intermediaries, which stimulate proteolytic cleavage and release of membrane-tethered growth factors that bind and thereby transactivate EGFR. Specifically, estrogen binding to the GPCR GRP30 facilitates matrix metalloproteinase–2 and –9 (MMP2 and MMP9) cleavage of the growth factor precursor pro-HB-EGF, thus initiating HB-EGF–mediated EGFR transactivation.177 Through a related mechanism, LPA-, SIP-, and thrombin-activated GPCRs transactivate EGFR in breast cancer cells via growth factor shedding of tumor necrosis factor (TNF)-α through the action of TACE/ADAM17 zinc-dependent proteases.179 Thrombin-mediated N-terminal cleavage and activation of proteinase-activated receptor 1 (PAR1), which can act through EGFR, has been found to promote metastasis and invasiveness in melanoma, breast, colon, and prostate cancers.133,134 Intriguingly, MMP1 was found to function similarly to thrombin in activating PAR1 and promoting breast cancer tumorigenesis and invasion.180,181 This cross talk between GPCRs and EGFR provides a rationale for the combinatorial use of GPCR agonists/antagonists and EGFR inhibitors in patients with EGFR-driven lung and colorectal cancers (see the RTK section). Aberrant GPCR signaling through Gα12/13 also contributes to tumorigenesis by enhancing cancer cell migration, invasion, angiogenesis, and metastasis.182 Ligand-activated LPA, PAR1, SIP, thromboxane A2 (TP), CXC chemokine (CXCR4), and prostaglandin E2 receptors couple to Gα12/13 and RhoGEFs to hyperactivate RhoA (described previously), which elicits these progression-associated phenotypes in glioma, melanoma, lung, breast, and ovarian cancers.133,134 Overexpression of Gα12/13 and RhoA in breast, prostate, and colon cancers also promotes metastasis by decreasing cell adhesion.165 RhoGEF inhibitors, RhoGTPase inhibitors, inhibitors of prenylation that could indirectly impair proper Rho localization (e.g., statins and farnesyl/geranylgeranyl transferase inhibitors), and inhibitors of kinases downstream of Rho (e.g., ROCK, LIMK, MRCK, and PAK) have all been developed and tested in biochemical, cell line, and mouse experiments; however, a clear benefit to the use of such compounds in patients with cancer has yet to be established.183 Two other prominent examples of dysregulated GPCR signaling in human cancer are the Hh/Smoothened (SMO) and Wnt/Fz signaling pathways.132,133,184 Secreted Hh ligands (first identified based on their roles in normal development and stem cell homeostasis, with sonic hedgehog [SHH] being the most ubiquitous) bind to the 12-pass transmembrane receptor Patched (PTCH), which relieves its repression of the GPCR SMO.185 Activated SMO couples to Gαi and Gα12, which regulate the glioma-associated oncogene homologue (GLI) transcription factors, which in turn regulate proliferative, survival, and differentiation signals involving cyclin D1, MYC, BCL2, and the forkhead transcription factors, to name a few.186,187 Mutations in SHH, PTCH, and SMO are found in patients with inherited and sporadic basal-cell carcinomas,188,189 whereas overexpression of Hh ligands have been shown to result in hyperactivation of the pathway in breast, colon, prostate, and pancreatic ductal adenocarcinomas.184,190 Vismodegib (GDC-0449), a selective SMO receptor inhibitor, was approved in January 2012 for the treatment of advanced basal-cell carcinomas (Table 2-1).191,192 Several other Hh/SMO inhibitors are currently being tested in patients with cancer, mostly for advanced and/or metastatic solid tumors, namely saridegib (IPI-926), BMS-833923 (XL139), LEQ506, LDE225, PF-04449913, and TAK-441.133,193 The secreted Wnt glycolipoprotein ligands activate the single transmembrane low-density lipoprotein related coreceptors LRP5/6 and the GPCR-like transmembrane protein Fz, which are phosphorylated and likely couple to Gαq and Gαo, respectively, to activate the cytoplasmic scaffold Dishevelled.196–196 Dishevelled in turn inhibits the β-catenin degradation complex (which consists of adenomatous polyposis coli (APC), axin, CKI-α, and GSK-3β, and the E3 ubiquitin ligase β-TrCP).197 This inhibition results in accumulation of β-catenin, which translocates to the nucleus, where it induces T-cell factor/lymphoid enhancer factor (TCF/LEF)-mediated transcription of genes important for cell differentiation and proliferation, including myc, cyclin D1, VEGF, FGF4/18, E-cadherin, COX2, and members of the Wnt cascade itself.196,198,199 Noncanonical Wnt/Fz pathways include signaling to the transcription factors nuclear factor of activated T cells (NFAT) via Gαq/i/Gβγ/PLC/PKC/Ca+ (Wnt-calcium pathway) and activator protein–1 (AP1) via RHO/RAC/JNK (Planar cell polarity pathway).132,200 These pathways regulate cell polarity and migration and are implicated in cancer metastasis.200 Aberrations in canonical Wnt signaling promote tumorigenesis in melanoma, colon, liver, ovarian, and prostate cancers.201 Specifically, loss-of-function mutations or truncations in APC and AXIN1/2 and gain-of-functions mutations in β-catenin are found in almost all colorectal cancers, with APC alteration found in more than 85%.196,202 Germline mutations in the APC gene are also the basis for the inherited cancer predisposition syndrome familial adenomatous polyposis (FAP).203 Efforts to develop selective inhibitors of Wnt signaling are ongoing and will be aided by current endeavors to crystallize members of the Wnt cascade.204 Of note, nonsteroidal anti-inflammatory drugs (NSAIDs) have shown some promise in modulating Wnt signaling, likely by inhibiting the Wnt-output gene COX2 or by enhancing E-cadherin signaling.196,205 NSAIDs are approved by the FDA for the treatment of patients with FAP.204,206 Cytokines are protein and glycoprotein ligands secreted by immune cells that initiate diverse and often opposing effects based on target cell lineage. Processes regulated by cytokine signaling include cell proliferation, differentiation, survival, inflammation, angiogenesis, antiviral activity, and modulation of immune function. Cytokines signal in an autocrine and/or paracrine fashion and can be subclassified by protein structure into four families, which total more than 100 members: hematopoietins, interferons (IFNs), chemokines, and the tumor necrosis factor (TNF) superfamily. The hematopoietin family consists of interleukins (IL-1 to IL-31), growth hormone, prolactin, erythropoietin, thrombopoietin, leptin, granulocyte and granulocyte-macrophage colony stimulating factor, and a few others.207 The majority bind to either class I or II cytokine receptors, which are single transmembrane glycoproteins that lack kinase activity and diverge in their extracellular domains to specify ligand binding.208,209 Class I receptors function as a cluster of two or three subunits that each have two sets of conserved cysteine pairs and a WSXWS motif in their external domains. Class II receptors lack the WSXWS motif and one of the class I conserved cysteine pairs, but they contain conserved proline, tryptophan, and an additional two conserved cysteine residues. Ligand binding causes aggregation of the γ chain (also called γc, CD132), which is common to many cytokines, and the β chain (also known as IL-2R-β, IL-15R-β, or CD122).210,211 In the case of specific cytokines, such as IL-2, association with a third subunit, the α chain (also called IL-2R-α, CD25, or Tac antigen), allows for high-affinity ligand binding.212,213 The IFN family members are divided into types I, II, and III classes.214,215 Most IFNs are type I and can be further subtyped.216 All type I IFNs bind the type I IFN receptor, which consists of two subunits (IFNAR1 and IFNAR2). The sole type II IFN, IFN-γ, binds the type II IFN receptor, which is composed of the IFNGR1 and IFNGR2 subunits. Both types of IFN receptors belong to the class II cytokine receptor family. The IFN family also includes a third branch, the IFN-like molecules, IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B), which display some structural overlap with interleukins and the antiviral properties of IFNs and bind a distinct receptor made up of IFNLR1/IL-28R-α and IL-10R-β.217 Given that cytokine receptors are promiscuous and bind multiple cytokine ligands, there is a high degree of redundancy in the output profiles of individual cytokines. This redundancy serves to amplify and sustain signaling downstream of these transitory stimuli. Hematopoietin or IFN binding induces oligomerization of cytokine receptor subunits and autophosphorylation and activation of the Janus activated kinase (JAK) family of intracellular tyrosine kinases (TYK2, JAK1-3), which are constitutively bound to box I and box II α-helix motifs in the receptor cytoplasmic tail.218 Activated JAK proteins phosphorylate tyrosine residues on the cytokine receptor chains, which classically bind the STAT family of transcription factors (STAT1-6).207,219 Docking of STATs facilitates their phosphorylation by JAKs, which in turn causes STAT dimerization, nuclear translocation, and alterations in gene transcription. There is also cross talk between STAT signaling and the NF-κB and SMAD signaling pathways.220 STATs are also activated by receptor tyrosine kinases, SRC, and ABL, and STAT activation also plays a role in transformation initiated by these oncogenes.221 JAK/STAT activity is regulated by several posttranslational modifications, as well as by the suppressor of cytokine signaling (SOCS) and protein inhibitor of activated STAT (PIAS) proteins.220 In addition to JAK-STAT signaling, cytokine receptors can transduce messages through LCK and SYK (Src-family kinases), BCL2, and via PI3K/AKT– and RAS/RAF–mediated upregulation of FOS- and JUN-dependent transcription.221,222 The 29-member TNF superfamily of receptors and their corresponding 19 ligands induce inflammation, in addition to prosurvival and proapoptotic phenotypes.223,224
Intracellular Signaling
Receptor Tyrosine Kinase Signaling
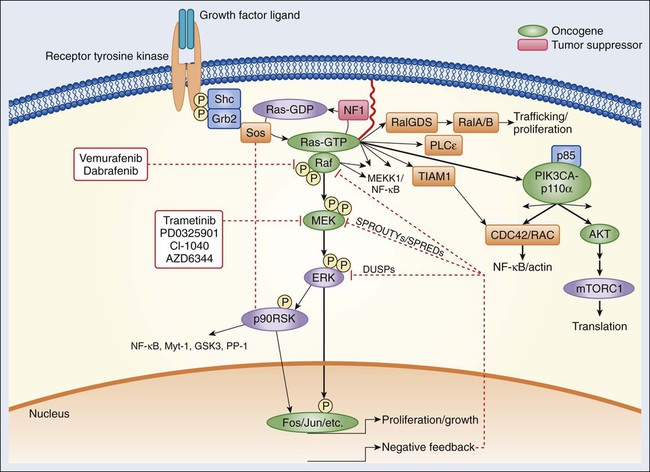
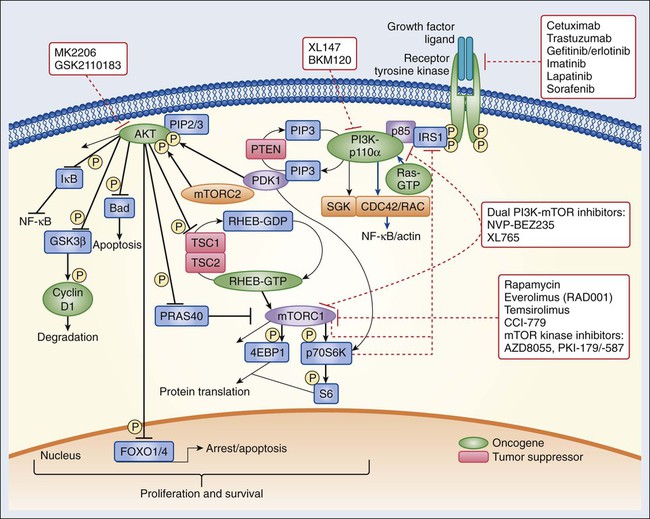
Epidermal Growth Factor Receptor Signaling
Molecular Target
Drug
Type of Compound
Approval Year/Trial Stage
Indications
ALK
Crizotinib
TKI
Aug 2011
NSCLC (ALK+)
Phase 2
ALCL (ALK+)
Phase 2
Local/Advanced Tumors (ALK mutant)
AR
Bicalutamide
NS-AR-CI
Oct 1995
Prostate Cancer
Enzalutamide
NS-AR-A
Aug 2012
Prostate cancer
AKT
MK2206
AI
Phase 2
Solid tumors
GSK-2110183
KI
Phase 2
Hematologic/Solid Tumors
BCR-ABL
Imatinib
TKI
May 2001
CML (Ph+)
Oct 2006
ALL (Ph+)
Dasatinib
TKI
June 2006
CML (Ph+)
June 2006
ALL (Ph+)
Nilotinib
TKI
Oct 2007
CML (Ph+)
Bosutinib
TKI
Sept 2012
CML (Ph+)
BRAF
Vemurafenib
KI
Aug 2011
Melanoma (BRAF mutant)
Phase 3
Thyroid cancer
Phase 1
Colon cancer
Dabrafenib*
KI
Phase 3
Melanoma (BRAF mutant)
EGFR
Gefitinib
TKI
May 2003
NSCLC (EGFR mutant)
Erlotinib
TKI
Nov 2004
NSCLC (EGFR mutant)
Nov 2005
Pancreatic cancer
Cetuximab
mAb
Feb 2004
Colorectal cancer (EGFR+)
March 2006
HNSCC
Panitumumab
mAb
Sept 2006
Colorectal cancer (EGFR+)
ERBB2
Trastuzumab
mAb
Oct 1998
Breast cancer (HER2+)
Oct 2010
Gastric Cancer (HER2+)
Lapatinib
TKI
March 2007
Breast cancer (HER2+)
Pertuzumab
RTK-A
June 2012
Breast cancer (HER2+)
FLT3
Midostaurin
TKI
Phase 2
ALL (FLT3 mutant)
AC220
TKI
Phase 2
ALL (FLT3 mutant)
JAK2
Ruxolitinib
TKI
Nov 2011
Myelofibrosis (PV/ET)
SAR302503
TKI
Phase 2
Myelofibrosis (PV/ET)
KIT
Imatinib
TKI
Feb 2002
GIST
Sunitinib
TKI
Jan 2006
GIST
MEK
Trametinib*
KI
Phase 3
Melanoma (BRAF mutant)
Selumetinib
KI
Phase 2
Melanoma (BRAF mutant)
mTORC1
Everolimus
SMI
March 2009
Renal cell carcinoma
May 2011
Pancreatic neuroendocrine tumors
April 2012
Renal angiomyolipoma (with TSC)
July 2012
Breast cancer (ER pos, HER2 neg)
Temsirolimus
SMI
May 2007
Renal cell carcinoma
PI3 kinase
BKM120
KI
Phase 2
Solid tumors
XL147
KI
Phase 2
Hematologic/solid tumors
PI3 kinase/mTOR
NVP-BEZ235
KI
Phase 2
Solid tumors
XL765
KI
Phase 2
Solid tumors
PDGFRα/β
Imatinib
TKI
Oct 2006
CMML (TEL-PDGFRβ fusion)
Oct 2006
HES (PDGFRβ fusion)
Oct 2006
DFSP
RET
Vandetanib
TKI
April 2011
Thyroid cancer
Sorafenib
TKI/KI
Phase 3
Thyroid cancer
Cabozantinib
TKI
Phase 3
Thyroid cancer
Motesanib†
TKI
Phase 2
Thyroid cancer
Smoothened
Vismodegib
GPCR-A
Jan 2012
Basal cell carcinoma
VEGF
Bevacizumab
mAb
Feb 2004
Colorectal cancer
July 2009
Renal cell carcinoma
Ziv-aflibercept
FP
Aug 2012
Colorectal cancer
VEGFR
Sorafenib
TKI/KI
Dec 2005
Renal cell carcinoma
Nov 2007
Hepatocellular carcinoma
Phase 3
Thyroid cancer
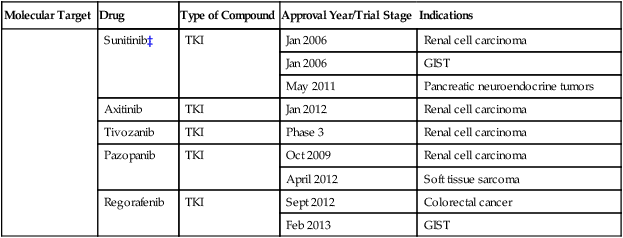
Sunitinib‡
TKI
Jan 2006
Renal cell carcinoma
Jan 2006
GIST
May 2011
Pancreatic neuroendocrine tumors
Axitinib
TKI
Jan 2012
Renal cell carcinoma
Tivozanib
TKI
Phase 3
Renal cell carcinoma
Pazopanib
TKI
Oct 2009
Renal cell carcinoma
April 2012
Soft tissue sarcoma
Regorafenib
TKI
Sept 2012
Colorectal cancer
Feb 2013
GIST
Insulin and Insulin-Like Growth Factor–1 Receptor Signaling
Platelet-Derived Growth Factor Receptor Signaling
Fibroblast Growth Factor Receptor Signaling
RET Signaling
Vascular Endothelial Growth Factor Signaling
Hepatocyte Growth Factor Receptor Signaling
G-Protein Coupled Receptors Signaling
Cytokine Receptor Signaling
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine