Fig. 9.1
The Melzack–Wall gate theory of pain. Thick myelinated afferent fibers and thin unmyelinated fibers synapse on projection neurons in the dorsal horn as well as inhibitory interneurons. A proportional increase in thick afferent fiber activity stimulates the inhibitory interneuron and closes the “gate” to afferent nociceptive signaling from the thin unmyelinated fibers
While clinical effectiveness in decreasing pain scores in a number of chronic pain states has been shown, the mechanism of action for analgesia has yet to be fully elucidated. One proposed mechanism of action relies upon the “gate control” theory of pain articulated by Melzack and Wall. According to the gate control theory the stimulation of large diameter Aβ fibers, which carry light touch sensation, will inhibit the transmission of afferent nociceptive input from smaller C-fibers and Aδ-fibers [2]. This modulation of nociceptive transmission occurs in the dorsal horn of the spinal cord. Another proposed mechanism suggests that SCS leads to the activation of inhibitory interneurons in the dorsal horn causing a release of acetylcholine and gamma-aminobutyric acid (GABA) that decreases the excitability of the spinothalamic tract projection neurons. Finally, another mechanism by which SCS may provide pain relief is via peripheral vasodilation. It is thought that this occurs by the antidromic activation of sensory afferent fibers, leading to a release of vasoactive substances in addition to a direct inhibition of sympathetic efferent activity leading to vasodilation [2]. The clinical effect of SCS is to attenuate the perception of neuropathic pain without changing the perception of acute nociceptive pain.
In a study performed by de Vos et al. [3], 11 patients underwent spinal cord stimulation who were diagnosed with diabetes and chronic neuropathic pain which had been unresponsive to conventional therapy. They had been treated with antidepressants, anticonvulsants, and opioids and had failed other therapies such as transcutaneous electronic nerve stimulation, physical therapy, and pain psychology counseling. All patients first had a percutanous trial of SCS. Of the 11 patients, 9 achieved >30% pain reduction on a visual analog scale (VAS) during the trial and subsequently went on to permanent stimulator implantation. The average VAS score was 77 at baseline. This decreased to an average score of 34 at 6 months, 23 at 12 months and 23 again at 30 months. In addition, they observed a significant decrease in pain medication usage in eight of nine patients with six of the eight relying solely upon SCS for managing their pain symptoms. Another study by Daousi et al. [4] evaluated patients with painful DPN who were treated with SCS and found very similar results with regard to reduction in VAS scores with stimulation. In the Daousi study the pain reduction was sustained at 7.5 years post implantation. A third study by Tesfaye et al. [5] once again showed a significant decrease in VAS scores with SCS when compared to no stimulation. Each of these studies showed approximately a 66% decrease in VAS scores compared to baseline with the use of spinal cord stimulation thus showing that SCS is an effective and viable option for the treatment of painful DPN.
Procedure Description
Prior to permanent implantation of a SCS device, the patient must first undergo a psychiatric evaluation to rule out major psychopathology or other factors that may limit the efficacy of SCS. Next the patient undergoes a trial of SCS for several days in order to determine whether or not the stimulator will provide significant analgesia. The trial involves the percutaneous placement of wire electrodes into the epidural space. This is performed in an outpatient procedure suite under fluoroscopic guidance. The wires are then connected to an external generator and programmed to produce non-painful, tingling paresthesias overlapping with the patient’s areas of pain. At the end of the trial these electrodes are removed in the office and results are evaluated. If the patient has significant reduction in pain and functional improvement, they may proceed with permanent implantation (Fig. 9.2).
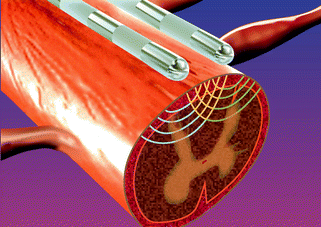

Fig. 9.2
Spinal cord stimulator implanted electrodes within the epidural space. The spinal cord stimulator leads are inserted at the L1–2 level and advanced within the epidural space to the desired vertebral level. The field generated by the electrodes penetrates into the dorsal spinal cord to stimulate dorsal horn neurons and ascending afferent fibers within the dorsal columns
Permanent implantation is performed in an outpatient surgical center, often with just local anesthesia and mild to moderate sedation. The process of percutaneous lead placement is similar to the trial implant. However, once the leads are properly positioned and adequate stimulation paresthesias are achieved, a skin incision is made over the spine and the leads are anchored to the interspinous ligament or dorsolumbar fascia to prevent movement of the wires in the epidural space. A subcutaneous pocket for the generator is then created in the flank or superior gluteal region and the leads are tunneled subcutaneously from the paraspinal incision to connect to the generator.
Procedure Complications
The complications associated with SCS are primarily hardware related or surgical, with the most common complication being electrode migration (27%). Other complications include cerebrospinal fluid leaks (7%), infection (6%), and battery failure (6%) [6]. In addition, there have been case reports of epidural hematoma formation leading to neurologic dysfunction and even paralysis [7].
High Concentration Capsaicin Dermal Patch
The capsaicin 8% topical patch (Qutenza) is a recently FDA approved treatment for painful post-herpetic neuralgia. While its use in the treatment of DPN is considered off-label, its efficacy in improving pain scores in patients with peripheral neuropathic pain, including DPN, has been shown in a few studies. Although the 8% topical capsaicin patch is classified by the FDA as a transdermal drug, it must be applied in a physician’s office as a one-hour treatment. For this reason we have included it along with other interventional therapies.
High concentrations of capsaicin applied topically produce pain via activation of the transient receptor potential vanilloid receptor 1 (TRPV-1) [8]. This pain is perceived as a burning sensation. There are a number of over-the counter preparations of topical capsaicin cream. These come in various concentrations ranging from 0.025 to 0.1% capsaicin and are meant for daily use. In contrast, a physician applies the capsaicin 8% topical patch approximately once every three months. In order to minimize the discomfort associated with capsaicin application, the patient is pretreated with a topical anesthetic in the planned treatment area along with oral analgesics [9].
The mechanism of action for analgesia with topical capsaicin appears to be the drugs affinity for the TRPV-1 receptor, a ligand-gated, nonselective cation channel [8]. Altered levels of expression of this receptor on small-diameter afferent nociceptive neurons (C- and Aδ-fibers) have been shown in patients with DPN [10]. The TRPV-1 receptor is both sodium and calcium permeable with a calcium-to-sodium permeability ratio that at baseline is about 8:1 but can increase to 25:1 with prolonged activation [11]. The prolonged activation leads to a rapid and sustained influx of calcium into the nerve fibers, which then causes the breakdown of cytoskeletal components such as microtubules. This leads to a reduced responsiveness of the nerve fiber. In addition, there is a reversible regression of the nerve fibers terminals that can be seen histologically as a loss of nociceptive nerve fiber density in the dermal and epidermal layers [12]. The combination of decreased nerve fiber responsiveness as well as nerve fiber regression results in an overall inhibition of nerve pain transmission.
An open-label study by Webster et al. [13] enrolled 117 patients with peripheral neuropathic pain, of which 91 patients had DPN, to evaluate the safety, efficacy, and tolerability of the 8% capsaicin dermal patch. Prior to the application of the patch, each patient was pretreated for 60 min with a lidocaine 4% topical anesthetic. The patch was then applied once for either 60 or 90 min. Patients recorded pain scores on a daily basis throughout the 12 week study period. In addition, patient global impression of change (PGIC) was evaluated at weeks 2, 6, and 12. The application of the capsaicin patch was well tolerated but approximately half of the DPN patients required additional analgesia in the form of one hydrocodone 5/500 mg tablet during the application. The patients with DPN reported a mean 31.4% reduction in pain during weeks 2–12 with 47% of patients experiencing a ≥30% decrease in pain from baseline scores. At week 12, 32% of patients reported their PGIC scores as much or very much improved. The results for the 60 min and 90 min applications were comparable.
Procedure Description
Prior to patch application the patient should be treated with an oral analgesic such as hydrocodone or oxycodone. The physician will then mark skin to outline the painful areas to be treated. The treatment area is then washed with soap and water and dried. A topical anesthetic such as lidocaine 4% cream can be applied to the area prior to patch application. Once the skin is anesthetized, the topical anesthetic must be removed and the area washed and dried again. The patch or multiple patches are then cut to fit the demarcated area on the patient’s body. The self-adhesive backing is exposed and the patches are applied for one hour. The patches are wrapped to avoid exposing non-treated skin. While handling the patches the physician must wear gloves, and during treatment the patient should avoid touching the patch. After patch removal the treated area is cleansed with a solution supplied with the patches to remove residual capsaicin. Due to the risk of eye injury it is not recommended to treat areas above the neck [14].
Procedure Complications
The primary complications related to the application of the capsaicin 8% patch are local, transient, application site reactions. Patients may experience erythema and pain over the areas being treated [11]. Occasionally the pain may be severe enough to require early termination of treatment although with adequate pretreatment with oral analgesics and/or topical anesthetics this is not common. Treatment with topical capsaicin does not produce cutaneous anesthesia and has not been shown to decrease sensory function in patients with painful DPN [13].
Intrathecal Therapy
Intrathecal (IT) drug delivery is generally reserved for patients who do not receive adequate pain relief or who experience dose limiting side effects with systemic drug therapy. The IT delivery of medication may be better tolerated than oral administration because lower doses are required and the drug is delivered directly to the site of action in the spinal cord, resulting in fewer systemic effects [15].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




