Interventional Approaches to Pain
Andrew Mannes
Philip S. Kim
Russell R. Lonser
Interventional and neurosurgical procedures can be utilized to supplement pharmacologic and complementary approaches in the treatment of pain (see Chapters 3 and 4). Pharmacologic therapies are described elsewhere in this text, including the principles of analgesic management using opioid agents and other adjuvant medications. The primary indications for interventional techniques are either for patients whose pain is poorly responsive to systemic analgesic therapies or for patients who suffer from intolerable side effects, in whom efforts to manage adverse effects are unsuccessful including patients experiencing severe dose-limiting side effects that prevent optimal titration to therapeutic levels (e.g., systemic opioids associated with refractory constipation, nausea, vomiting, or sedation).
Patient’s response to analgesic medicinal therapies has been best described in the cancer population. The oral administration of analgesics based on recommendations, including those outlined by the World Health Organization, can provide satisfactory relief to many patients. However, poorly relieved pain that is experienced by 5% to 15% (1,2,3) of the approximately 500,000 patients that die each year from cancer indicates a significant need for additional methods for control of pain, including interventional and neurosurgical procedures, that can offer symptom relief. Aside from optimizing pain control while minimizing side effects, interventional pain therapies can also enhance functional abilities and physical and psychological well-being, enhancing the patient’s quality of life (4). It has also been reported that better pain management utilizing interventional techniques may result in increased life. Further, reducing patient visits for symptom management could potentially reduce costs (5).
INITIAL EVALUATION
For the interventionalist, it is important to understand the patient’s prognosis, associated comorbidities, as well as the expectations of patient and family. An initial evaluation for interventional pain therapies should ascertain the patient’s general medical condition along with the primary disease. A complete history is required that includes a general medical, disease-specific (e.g., patients with oncologic disorders need to be thoroughly evaluated for possible local recurrence or new metastases), psychosocial, and pain histories. The physical examination includes a general medical examination with emphasis on neurologic findings. Examination of the site of pain and surrounding anatomic regions is critical (e.g., if a patient has profound motor and sensory deficits in a particular region, neurolysis techniques become a more acceptable therapeutic option). Specific pain history includes the following: quality of pain, pain intensity, alleviating/exacerbating factors, temporal characteristics, and duration and associated features (e.g., numbness, weakness, and vasomotor changes). Psychosocial evaluation should assess the presence of psychological symptoms (e.g., anxiety and depression), and psychiatric disorders (e.g., major depression and delirium) should be similarly addressed. The nature and meaning of the presenting pain needs to be distinguished from anxiety and suffering affecting the patient. The ability to cope and the availability of psychosocial support systems need to be assessed and reinforced with proper health and social professionals. A final assessment should determine the patient’s expectation of therapeutic interventional options.
Appropriate selection of an intervention is based on therapeutic goals. If the presenting pain is expected to be transient (pain that will be alleviated by primary radiation therapy or chemotherapy, pain that is associated with the treatment of the primary disease, or pain that is), then the intervention should likewise be reversible. However, if the pain is expected to be chronic, a technique that results in more permanent effects is indicated. Life expectancy must be considered when selecting an appropriate intervention. If the patient’s life expectancy is short, treatment strategies should strive to minimize the frequency and level of interventions and recovery time and should focus on optimizing a patient’s quality of life. A patient with a longer life expectancy may warrant more extensive and expensive interventions (i.e., implantable devices). Certain procedures may not be indicated for patients with longer life expectancy such as neuroablative procedures that are associated with permanent loss of function or a theoretical risk of developing deafferentation pain syndromes.
Once a definitive diagnosis of the cause of the underlying source of pain has been made, a treatment plan should characterize the expected outcome, define contingencies, and plan for reassessment. Longitudinal monitoring of pain and response to interventional therapies is essential and allows implementation of additional options (e.g., complementary therapies, pharmacologic strategies, and behavioral and psychological approaches). This chapter includes some of the frequently utilized procedures in the palliative care pain population (Table 4.1). Not all indications and contraindications are included and consultation with a pain practitioner should be considered before referring the patient for evaluation and treatment.
TABLE 4.1 Comparison of interventional pain procedures | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
APPROACHES TO INTERVENTIONS
Pharmacologic management of pain can be viewed as a continuum of indirect and direct drug delivery paradigms (4). Indirect drug delivery (i.e., systemic analgesia) refers to the administration of an analgesic into the bloodstream, which is then transported to the receptor site in neural tissue:
By systemic absorption
By formulation of depot for sustained and continuous release
Through the bloodstream
Direct drug delivery is the administration of an agent to the targeted neural tissue involved in nociception. By delivering directly to the nociceptive pathways, a pronounced analgesic effect at a lower dose with fewer side effects can be achieved. An example of this is comparing equianalgesic morphine doses in the intrathecal, epidural, and intravenous spaces (Table 4.2) (6).
Interventional pain therapies are often minimally invasive techniques that can be categorized into direct drug delivery, neuroablation, neural blockade, and neurostimulation. Direct drug delivery involves the administration of analgesics, usually opioids and local anesthetics, regional to nociceptive pathways. Other potential agents such as α2-agonists and calcium channel blockers can be administered. Neuroablation refers to direct chemical, thermal, or surgical destruction of nociceptive pathways. Neurostimulation or neuroaugmentation refers to the application of direct electrical stimulation to inhibit nociceptive transmission. Not all pain, however, can be adequately addressed using these techniques. In such cases, one can consider consultation with a neurosurgeon about surgical intervention.
TABLE 4.2 Equianalgesic morphine conversions among routes of administration | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Direct Drug Delivery
Neuraxial direct drug delivery involves accessing the epidural or subarachnoid (intrathecal) space by a needle and/or the placement of a continuous infusion system. In general, neuraxial infusion should be considered when severe pain cannot be controlled with systemic drugs and/or because of dose-limiting toxicities. Neuraxial infusions can also be considered when there is an immediate need for using various nonopioid analgesics. Specifically, local anesthetics can have a profound analgesic effect on many intractable opioidunresponsive pain conditions. Although it is possible to give local anesthetic systemically, higher local concentrations can be achieved, resulting in profound neural blockade through direct drug delivery.
Neuraxial delivery systems have two components: a subarachnoid or epidural catheter and a delivery mode (e.g., bolus dosing, syringe pump, internal port, or internal or external pump). There are basically five types of neuraxial drug delivery systems, and familiarization with these systems allows the clinician to understand the respective advantages and disadvantages of each (7,8).
The simplest, least expensive, and least invasive option is a percutaneous catheter, which is typically made of nylon, polyurethane, or polyamide and can be wire reinforced. These catheters are routinely placed in surgical and obstetric
patients to manage operative and postoperative pain and are designed for short-term use (generally <1 week). But a catheter may be maintained for longer periods without problems and may suffice for the duration of the patient’s life. If there is a complication, these catheters can be discontinued by removing the dressing and withdrawing the catheter. Occasionally, these catheters can cause a localized tissue reaction at the site of insertion, can migrate, and are susceptible to accidental displacement.
patients to manage operative and postoperative pain and are designed for short-term use (generally <1 week). But a catheter may be maintained for longer periods without problems and may suffice for the duration of the patient’s life. If there is a complication, these catheters can be discontinued by removing the dressing and withdrawing the catheter. Occasionally, these catheters can cause a localized tissue reaction at the site of insertion, can migrate, and are susceptible to accidental displacement.
The next type of drug delivery system uses the same type of catheter as that mentioned in the preceding text, but it is tunneled subcutaneously to decrease the incidence of migration. Placement can be performed in a clinic and requires a small incision with multiple needle insertions. Tunneling the catheter is better suited for the outpatient or the homebound patient.
Implanted catheters with subcutaneous injection site are technologically more advanced and require a minor surgical procedure, resulting in higher costs (for placement). Sterile preparation and the use of fluoroscopy are essential. These systems can be placed in the epidural or intrathecal space. There are two basic designs: exteriorized or completely internalized injection port. In the first design, the proximal catheter is tunneled from the exit site in the back and exteriorized usually along the midaxillary line. This catheter can include an antimicrobial cuff that reduces both infection and catheter migration. In the second design, the port is supported by bone, usually a rib, so as to facilitate needle insertion. It can be used for intermittent bolus dosing or accessed for continuous infusions.
A totally implanted catheter with implanted reservoir and manual pump is being developed by several companies, as well as an implantable patient-activated device. This design allows patient-controlled analgesia by pressing the activation valve and pumping chamber, providing a bolus of medication. Because the entire device is implanted, the reservoir is refilled by inserting a needle into a subcutaneous port in the control pad.
A totally implanted catheter with implanted infusion pump is available in two basic designs. The simpler design is a constant fixed infusion pump in which the dose can be adjusted by a clinician changing the concentration. The second type includes a programmable, peristaltic infusion pump with a drug reservoir, an electronic module, and an antenna allowing reprogramming of drug flow rates. The clinician controls the pump through an external programmer head (such as a pacemaker) to alter the dose, give single doses, or change the continuous infusion rate.
Recently, Medtronic has received U.S. Food and Drug Administration approval for a patient activation device that will allow the patient to receive a medical direct bolus of medication when the device is activated.
Typically, a percutaneous test catheterization of the epidural or intrathecal space would be performed to assess the efficacy and starting doses of medication before implanting a permanent delivery system. There are several approaches to trial the drugs, including bolus dosing, by accessing the intrathecal space with a spinal needle or by placement of a catheter and continuous infusion—either in the epidural or intrathecal space. Ideally, the method utilized for clinical assessment would best emulate the intended route (e.g., a trial with a continuous intrathecal catheter for evaluating future implantable pump placement). Although the complication rate is low, implantable devices can have problems with catheter failure, infection, seroma, wound dehiscence, and catheter tip fibroma formation (9). They also require health-care provider visits for routine refills and adjustment of dosing.
The selection of the appropriate neuraxial drug(s) and delivery system for an individual patient is based on several considerations (7,10):
Patient life expectancy
Economics and cost-effectiveness
Choice of epidural versus subarachnoid route of administration
Patient life expectancy and duration of need are difficult to predict. The more sophisticated implantable systems are expensive devices that require a trial catheter, adjustments of medications, and a surgical procedure for placement. One study by Bedder et al. suggests that an implanted pump system is a more viable financial alternative compared with other drug delivery systems for a period over 3 months (11). The less sophisticated, percutaneous and tunneled catheters are best suited for patients with a limited life expectancy of a few months. Both epidural and subarachnoid drug deliveries can be equally effective. The duration of therapy will usually predict the type of infusion system selected. Catheter obstruction, fibrosis, and loss of analgesic efficacy are well described in long-term epidural drug systems (7). Therefore, intrathecal drug delivery systems are best suited for a protracted duration of therapy (>3 months). A decision-making algorithm for using neuraxial analgesia is shown in Figure 4.1 (12).
Multiple pharmacologic preparations have been administered through the neuraxial drug delivery systems. The gold standard is morphine, which is widely used and successful. When intrathecal morphine provides inadequate relief, other opioids, such as hydromorphone, meperidine, methadone, fentanyl, and sufentanil, have been used. As tolerance develops, one might switch opioids and/or use them in combination with coanalgesics, which include local anesthetics (e.g., tetracaine and bupivacaine), α2-agonists (e.g., clonidine), and γ-aminobutyric acid (GABA-B) agonists (baclofen). Ziconotide, the first neuronal calcium channel blocker for pain, is the synthetic equivalent of a peptide produced by a snail and has been approved for use in intrathecal pumps for intractable pain not responsive to systemic analgesics including intrathecal morphine (13). Drug selection is based on the patient’s pain symptoms using clinical strategies that have been developed (e.g., comprehensive consensus-based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain updated 2011) (14). The guidelines and algorithms were developed by an expert panel, evaluating existing literature and algorithms for various
intrathecal drugs. The optimal drug dosage, concentration, and issues related to compounding of drugs have been reviewed.
intrathecal drugs. The optimal drug dosage, concentration, and issues related to compounding of drugs have been reviewed.
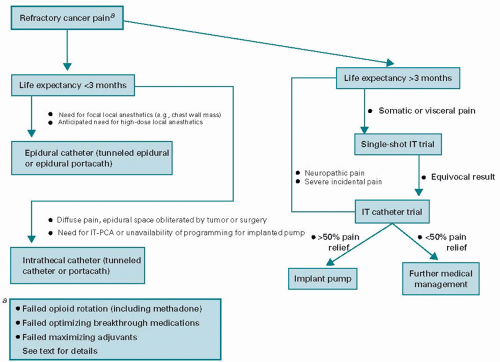 Figure 4.1. A decision-making algorithm for patients with refractory cancer-related pain. IT, intrathecal; IT-PCA, intrathecal patient-controlled analgesia. |
Complications from neuraxial catheter and pump placement may result from anatomic changes, infection, fluid collection, catheter migration, or device failure. Patients with suspected block of the subarachnoid circulation due to tumor extension or subarachnoid hemorrhage/arachnoiditis may have a poor response to the delivery of intrathecal analgesia. Evidence of an obstruction should be sought using magnetic resonance imaging or myelography to determine the level of obstruction. Retesting the efficacy of analgesia by placing the injectant proximal to the obstruction may yield improved analgesic response. Migration or fracture of the catheter should be suspected if the patient reports sudden changes in pain relief or if a fluid collection is seen at the insertion site. Percutaneously placed catheters can be bolused with a test dose of a local anesthetic to assess function. Myelography should be performed with implantable catheters or pumps (through a side port) to determine catheter placement and function when displacement or catheter rupture is suspected.
An infection of the site does not always necessitate immediate removal of a catheter. Superficial infections may only require a course of antibiotics. However, persistent or progressive tissue infection or central nervous system (CNS) involvement necessitates immediate removal of the catheter and/or pump.
A growing body of case reports and studies supports the benefits of direct drug (intrathecal) delivery systems. In a study of 202 patients experiencing refractory cancer pain who were randomized to receive either an implantable drug delivery system or comprehensive medical management (15), the patients receiving implantable intrathecal pump had reported successful pain control with a reduction in common drug toxicities such as fatigue and diminished level of consciousness. Overall, there was an improvement in the quality-of-life measures and survival over the 6 months in the patients receiving implantable intrathecal pump.
Peripheral Nerve/Plexus Drug Delivery
Blockade of peripheral nerves and neural plexi is commonly performed to provide regional anesthesia and analgesia to patients undergoing surgical procedures (15,16,17,18). Modified constant infusion systems typically deliver local anesthetics directly to peripheral nerves and neural plexi in patients with inadequate analgesia or intolerable toxicities from systemic medications. Specific localized pain syndromes related to a mononeuropathy, plexopathy, and peripheral neuropathy may benefit from peripheral nerve infusion.
Continuous neural blockade of the brachial plexus is common for postoperative pain. A technique of placing a catheter along the brachial plexus and self-contained infusion system has been described (16,17). A case report describes
the successful 2-week management of pain from pancoast syndrome with a brachial plexus infusion system using local anesthetics (19). Other potential areas where neural infusion could be performed include the lumbosacral plexus, paravertebral and selected peripheral nerves, and sympathetic chain.
the successful 2-week management of pain from pancoast syndrome with a brachial plexus infusion system using local anesthetics (19). Other potential areas where neural infusion could be performed include the lumbosacral plexus, paravertebral and selected peripheral nerves, and sympathetic chain.
The current self-contained peripheral nerves/plexi infusion system is a modification of neuraxial infusion systems (16,17,18,19,20). The advantages of this system are the simplicity and the minimal invasiveness of the placement of the system. However, the patient can experience a localized tissue reaction or catheter migration and has a risk of infection for implantations of >1 month’s duration. Implantable technology must be improved to provide a completely implanted infusion system for long-term continuous nerve/plexi analgesic infusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree