Fig. 10.1
Post-lumpectomy section of a laser treated breast cancer showing concentric zones of coagulated tumor, hyperemic ring, and zone of fat necrosis around the laser fiber
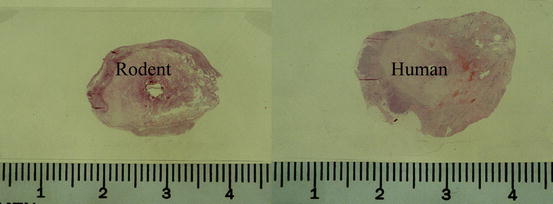
Fig. 10.2
Gross histology of laser treated tumors in a rodent tumor model and in a patient demonstrating 15–20 mm tissue necrosis caused by laser energy
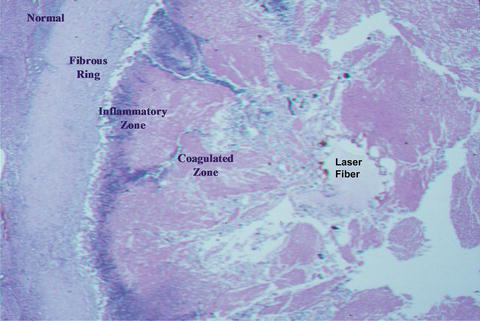
Fig. 10.3
Microscopic appearance of laser treated tumor at 1 month showing clearance of the coagulated tissue by inflammatory cells and formation of a fibrous ring
Experimental Basis for ILT
Mathewson et al. were probably the first to show the coagulation effect of Nd:YAG laser light delivered within a normal rat liver [9]. Employing low power (0.5–2.0 W) and long exposures of 1–40 min, they observed well-defined highly reproducible necrotic lesions of up to 16 mm in diameter consistent with tissue damage by a purely thermal effect. Radiological examination of the treated tissue in which arterial tree had been filled with a radio-opaque polymer demonstrated loss of all small and some larger vessels. These investigators, however, noted tissue charring around the fiber tip with higher (>2 W) power settings and corresponding fall in the laser light transmission. To overcome this problem and to shorten the operation time, Dachman et al. were able to show the coagulative necrosis of the pig liver by ultrasound-guided Nd:YAG laser at power setting of less than 5-W over 6–10 min duration [10].
In 1988, the author developed a technique which allowed the delivery of laser energy into tumors at higher powers (5–10 W) and in shorter time [11, 12]. Thus, a typical 1-cm tumor could be ablated in 10–15 min. This goal was achieved by dripping normal saline at 1–2 cm3/min para-axially to the laser fiber into the tumor. The small pool of fluid in front of the fiber tip prevents the heated tissue sticking to and defacing the fiber tip. This allows laser light transmission to a distance of 5–8 mm into the target tissue for coagulation. The volume of the normal saline dripped into the tissue during the treatment time is about 15–20 cm3; small enough to be easily absorbed by the body. The temperature at the point of the laser light emission is continuously monitored with a thermocouple soldered to the laser needle (Fig. 10.4). By adjusting the laser power and the rate of the saline drip, this temperature is not allowed to exceed 100 °C. The temperature at the periphery of the tumor is monitored with a second multi-sensor thermal needle inserted parallel to the laser needle and 1 cm away from it. Experimental observation on rodent mammary tumors treated with laser (unpublished data) revealed that when the peripheral temperature reaches 60 °C, 100 % tumor necrosis occurs.
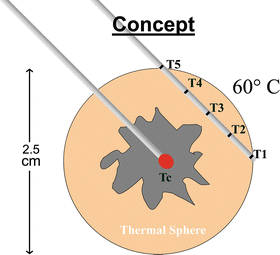

Fig. 10.4
A sketch of the laser probe tip in the center of the tumor and an adjacent multi-sensor thermal probe to continuously monitor the heating of the tumor
Clinical Experience with ILT
Liver Tumors
Most published reports related to clinical application of interstitial laser therapy are from investigators who treated primary or metastatic liver tumors with this technique. Hashimoto et al. first reported successful treatment of patients with hepatocellular and metastatic carcinoma of the liver treated intraoperatively with Nd:YAG laser given through a quartz fiber inserted into the tumor with ultrasound guidance [13]. There were no procedure-related complications. Schroder et al. reported similar experience in treating patients with liver tumors [14]. Nolsoe et al. reported successful interstitial laser treatment in 11 of 12 patients [15]. They employed multiple laser fibers with diffuser tips, sonographically guided into the center of tumors at 5 mm intervals through a template. Nd:YAG laser at power setting of 4–8 W was used. The periphery temperature reaching 60 °C during treatment was measured by micro-thermocouples. The treated tumors were noted to become hyperechoic. Follow-up ultrasound examination showed central cyst formation and needle biopsy revealed necrotic material. Patients experienced minor pain, transient pyrexia, and in one case pleural effusion which resolved spontaneously. Amin et al. reported on interstitial laser photocoagulation of 21 patients with 55 liver metastases using Nd:YAG laser at 2-W through a 200 μm bare-tipped fiber [16]. 100 % necrosis was achieved in 38 % of tumors as judged by ultrasound and CT scan. Their success rate was higher in tumors smaller than 4 cm in diameter. There were four cases of subcapsular hematoma and six cases of pleural effusion. The author treated 26 patients with primary and metastatic liver cancers, either as a sole treatment or in conjunction with surgical resection of the liver disease. It was concluded that ILT can successfully ablate tumors up to 25 mm. in diameter [17].
Malignant Breast Tumors
Harries et al. reported on interstitial laser photocoagulation of 44 patients with breast cancer treated with diode laser under local anesthesia [18]. Tumors measured 1–5 cm in diameter, were treated with ultrasound guidance in 42 cases and with CT in two cases. The investigators observed that greater tumor necrosis was achieved when there was tissue carbonization around the laser fiber tip and they confirmed the advantage of this effect by pre-charring the laser tip. There was no attempt at dosimetry or thermometry of the tumor during treatment. Real-time monitoring by ultrasound did not appear to predict the size of necrosis. Mumtaz et al. reported on 20 patients with breast cancer and noted that gadolinium-enhanced magnetic resonance imaging defined the extent of laser-induced necrosis and residual tumor after laser photocoagulation therapy [19]. Milne et al. measured the temperature changes in porcine tissue models using thermocouples inserted at various distances from the laser probe [20]. They concluded that interstitial laser thermotherapy produces symmetrical and predictable volumetric temperature increases.
The author’s experience at Rush University consists of two phases:
1.
From 1994 to 2001, the feasibility, efficacy and safety of ILT was tested in 54 patients with small (T1) breast cancers and periodically reported [11, 21, 22]. Patients underwent surgical excision of the laser treated tumors as part of the standard treatment of their breast cancers. All 54 patients underwent wire localization, surgical excision of the tumor and removal of the regional lymph nodes 1–8 weeks later. The blood flow to the tumor was redetermined with color Doppler ultrasound prior to excision (Fig. 10.5a, b). The overall success rate of the procedure including the learning phase and technical changes was 71 %. Under optimal operating conditions, total tumor necrosis was achieved in two series of 13 of 14 and 14 of 14 consecutive cases (96 %) [23].
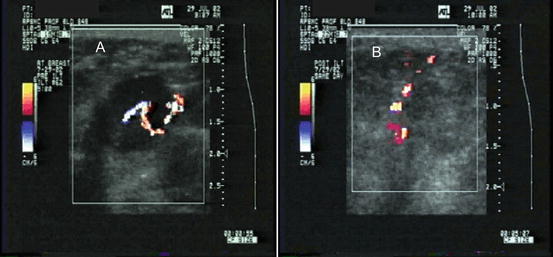

Fig. 10.5
Color Doppler ultrasound of a breast cancer treated with ILT showing a small vessel traversing the tumor before (a) and its disappearance due to thrombosis afterwards (b)
2.
Having gained technical proficiency and established the parameters for adequate treatment, the author treated selected patients, on volunteer basis, with laser and without excision. Over the next 4 years, ten patients with mammographically detected invasive breast cancers were treated with ILT without excision. The laser-treated tumors were monitored with imaging (ultrasound, mammography and MRI in one patient) and needle biopsy from marked areas at 1 month post-treatment (Fig. 10.6).
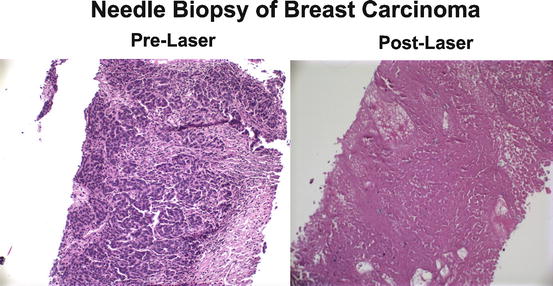

Fig. 10.6
Histologic appearance of needle biopsies of a breast cancer taken before and after treatment with laser
The mean tumor diameter as measured on mammogram and ultrasound was 11 (range: 7–21) mm. Patients required minimal oral analgesics and experienced no adverse systemic effects over a period of 1–12 years. Laser treated tumors became non-palpable after 3–6 months. Color Doppler ultrasound demonstrated loss of blood flow in the ablated zone and needle biopsies showed fibrosis. In three patients the laser- treated tumor became cystic (Fig. 10.7) and the aspirated fluid showed no malignant cells. In one patient, a 2 mm × 2 mm island of cancer, adjacent to an artery, was detected by ultrasound and confirmed by histological examination of the excised tumor (Fig. 10.8). This was deemed to be due to the heat-sink effect of the vessel. A multi-site prospective clinical trial to duplicate this experience is planned to commence in the near future.
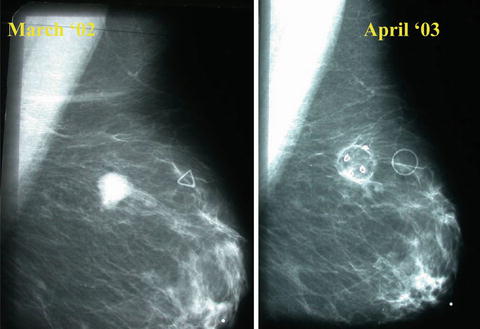
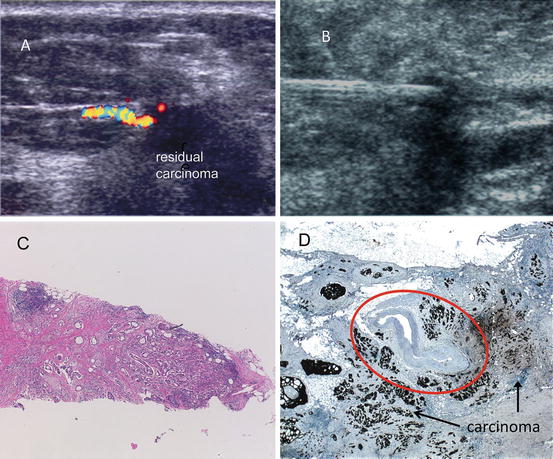

Fig. 10.7
Mammographic appearance of a breast cancer 1 year after laser therapy demonstrating conversion of the tumor into a cyst

Fig. 10.8
Detection of residual carcinoma: (a) Color Doppler ultrasound at 1 month after ILT. (b) US guided needle biopsy. (c) Needle core biopsy showing coagulated carcinoma on the left and residual malignancy on the right. (d) Malignant tissue seen surrounding the artery (circled)
It is emphasized that laser therapy of mammographically detected breast cancers is only to replace lumpectomy. All other components of the treatment, i.e., sentinel node/axillary node biopsy, radiation therapy to the breast and whenever indicated chemo-hormonal therapy is given per current standard of care.
Patient Selection
The following criteria are suggested for treatment of breast cancers with ILT:
1.
Clearly visualized masses or clusters of micro-calcifications detected by mammography (Fig. 10.9).
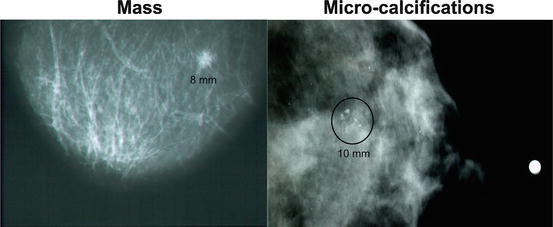

Fig. 10.9
Mammographically detected breast cancer as a mass or a cluster of micro-calcifications
2.
Tumor size: Up to 15 mm as determined by ultrasound and MRI.
3.
A distance of 1 cm should separate the tumor from the skin or the chest wall.
4.
Definitive needle core diagnosis of the tumor indicating: in situ or invasive cancer and determination of prognostic factors on the samples.
Pretreatment Evaluation
Imaging work up of the breast should include diagnostic mammography, grey scale and color Doppler ultrasound as well as contrast enhanced MRI. High resolution US provides very precise measurements of the tumor dimensions. Color Doppler US evaluates the blood flow in and around the tumor prior to intervention [11]. MRI excludes any secondary focus of cancer before laser therapy as well as any residual malignancy afterwards.
On the day of treatment, the tumor images are reviewed and its boundaries with all its visible extensions plus 0.5 cm of normal appearing tissue are marked on the films. This approach is also applicable to clustered microcalcifications associated with tissue densities. The volume of the tumor and 0.5 cm of the adjacent surrounding zone are calculated by V = 4/3⋅R3, R being the radius of the therapeutic sphere. Based upon the previous experimental data, the amount of laser energy needed for 100 % tumor coagulation is 1,400 J per cubic centimeter of the calculated tissue (tumor + surrounding breast parenchyma). Thus, for a typical 1.0 cm. tumor + 0.5 cm rim of surrounding parenchyma, the volume is 4.0 cubic cm and the laser energy for its complete destruction is 5,600 J. In practice, the treatment endpoint is reached when the thermal sensors on the needle adjacent to the tumor display 60 °C. The tumor blood flow is also assessed by contrast enhanced color Doppler ultrasound. This test is repeated after laser therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree