1
Reduction of adverse effects from chemotherapy and radiotherapy, with particular reference to nausea and vomiting
2
Management of pain
3
Reduction of adverse effects due to iatrogenic menopause
4
Improvement in quality of life
5
Resolution of anxiety, depression, fatigue; supportive treatment for chemotherapy and radiotherapy; perioperative noise reduction
6
Gastrointestinal disorders, sleep disorders
7
Prevention of relapse
8
Reduction of muscle disorders, palliative care, reduction of neuropathy
Table 25.2
Integrative oncology in Italy: ARTOI
Spreading of scientific knowledge |
Scientific research |
To integrate therapies and methodologies (hyperthermia) |
To develop personalized therapy (pharmaco and nutrigenomic) |
To improve results and quality of life |
Prevention |
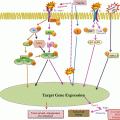
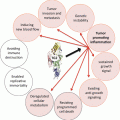
Our perspective must change from one of ‘the tumor in the patient’ to one of ‘the patient with the disease’ being at the center of our work. In order to achieve this, we must select chemotherapy tailored more specifically to the patient, more targeted biological therapy, and natural substances for improved synergy, as well as involving psycho-oncology to improve patient compliance. Although we know that many anticancer drugs come from natural substances (Table 25.3) and that the next generation of monoclonal antibodies may provide benefit at affordable costs, with numerous side effects so serious as to stop treatment (Table 25.4); however, medium- and long-term results are not yet well known. We know the immunomodulatory action and antineoplastic activity (Table 25.5) of many natural substances (Table 25.6). The dilemma is to understand whether sufficient scientific evidence exists to combine them with an antineoplastic drug. One of the most important factors is to understand the pharmacokinetics of substances. We know that the main pharmacokinetic mechanisms are related to absorption, distribution, metabolism, and excretion. Each of these mechanisms is important for optimum drug function: drugs must be ingested, inhaled, or infused into the body (absorption); they must be satisfactorily distributed to the various organs (distribution patterns); they must have a regular activation function (metabolism); and they must be regularly removed so as not to cause damage via accumulation (excretion). All these stages are important, but our main interest is in the metabolism of anticancer drugs and their interactions with natural substances that can affect induction and inhibition. An enzymatic induction action means that a substance may increase the metabolism of a drug, leading to reduced time in the bloodstream, and therefore a reduced pharmacological effect. Conversely, an enzyme inhibition action means that the drug will have reduced metabolism, much of it will remain in the bloodstream, with subsequent increased pharmacological effects, side effects and therefore toxicity. Whereas the enzymatic source of greatest interest is the cytochrome P450 (CYP) family and we know that 35 % of oxidation is carried out by CYP3A4, we also know that this enzyme metabolizes 70 % of anticancer drugs (Table 25.7). However, many natural substances have the same metabolic pathway as CYP, with inhibiting or inducing effects (Table 25.8). Therefore, we must be very careful when combining certain substances with chemotherapy (Table 25.9). We do know that many other substances interfere differently with anticancer drugs, increasing their action or reducing their side effects (Table 25.10).
Table 25.3
Orthodox chemotherapy: chemical substances synthesized in the laboratory
Extract of plants (taxanes) |
Products of bacteria or fungi (antracyclins) |
Alchilants substances (nitrosuree) |
Composits of minerals (platinum) |
Hormones |
Table 25.4
Monoclonal antibodies
Substance | Name | Cancer | Price E | % result | |
|---|---|---|---|---|---|
Erlotinib | Tarceva | Lung IV | 4.600 | 2 month | ML20650, EURTAC |
Gefitinib | Iressa | Lung IV | 3.600 | 1.5 month | fase III ISEL |
Cetuximab | Erbitux | Colon/head | 600 | 15/70 | CA225025-EMR 62 202-006 |
Trastuzumab | Herceptin | Breast-colon | 1.000 | 35/10 | MO16432-BO18225 |
Bevacizumab | Avastin | Breast-colon | 2.000 | 10 month | ECOG E2100-AVF2107g |
Rituximab | Mabthera | LNHodgkin | 2.600 | 50/2 year | M39021 |
Pazopanib | Votrient | Kidney-sarcomas | 1.800 | 3 month | VEG105192 |
Sunitinib | Sutent | Kidney IV | 2.600 | 6–9 month | Fase III EORTC |
Pertuzumab | Perjeta | Breast IV | 4.500 | 6 month | CLEOPATRA |
Lapatinib | Tyverb | Breast IV | 1.800 | 2 month | EGF100151 |
Panitumumab | Vectibix | Colon IV | 2.000 | No results | Study of 1.189 pz |
Ipilimumab | Yervoy | Melanoma IV | 60.000 | 2 month | Fase 3 MDX010–20 |
Table 25.5
Plants with immunomodulatory activity
Tinospora cordifolia | Arabinogalactan, a. glucano |
Whatania somnifora | Arabinogalactan |
Astragalus membranaceus | Beta-glucans |
Echinacea purpurea | Arabinogalactans, pectins |
Glycyrrhiza glabra | Pectins |
Panax ginseng | Arabinogalactans, pectins |
Viscum album | Arabinogalactans, pectins |
Aloe barbadensis | Acemannan |
Panax quinquefolium | Arabinogalactan: alfa glucan |
Lentinus edodes |
Table 25.6
Natural Substances with anticancer activity
Apigenin | Licorice |
N. acetil cisteine | Magnosalin |
Curcumin (turmuric) | Omega-3 fatty acids |
Epigallocatechin (green tea) | Polypodium leucotomos |
Inosytol | Quercetin
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|