Multiple new treatment options for metastatic colorectal cancer have been developed over the past 2 decades, including conventional chemotherapy and agents directed against vascular endothelial growth factor and epidermal growth factor receptor. Combination regimens, integrated with surgical approaches, have led to an increase in median survival, and a minority of patients with resectable disease can survive for years. Clinical decision-making therefore requires a strategic, biomarker-based multidisciplinary approach to maximize life expectancy and quality of life. This review describes systemic approaches to the treatment of patients with metastatic colorectal cancer, including integration with liver resection, other liver-directed therapies, and primary resection.
Key points
- •
Median survival has increased substantially in recent years to nearly 30 months in patients with metastatic colorectal cancer treated with contemporary regimens.
- •
All patients with metastatic disease should undergo evaluation for potential resectability and/or liver-directed therapy, which can significantly improve outcomes and potentially cure a minority of patients.
- •
Chemotherapy regimens in initial and subsequent settings should be accompanied by a targeted agent, unless specific contraindications exist.
- •
RAS status should be checked in all patients with metastatic colorectal cancer. Anti–epidermal growth factor receptor antibodies should be only used in patients with “extended” RAS wild-type tumors.
Introduction
Metastatic colorectal cancer is an important contributor to the public health burden of cancer-related mortality. An estimated 136,830 people in the United States will be diagnosed with colorectal cancer in 2014. Approximately one-fifth of these patients will have distant metastatic disease at the time of presentation. The spread of primary colorectal cancer can occur by lymphatic and hematogenous dissemination, as well as by contiguous and transperitoneal routes. The presence of right upper quadrant pain, abdominal distension, early satiety, supraclavicular adenopathy, or periumbilical nodules usually signals advanced metastatic disease. However, given extensive staging and surveillance protocols, it is also common to identify metastatic disease based on imaging studies. The first site of hematogenous dissemination is usually the liver, followed by the lungs and bone. An exception is rectal cancer, which may metastasize initially to the lung because the inferior rectal vein drains into the inferior vena cava rather than into the portal venous system.
Although the prognosis for patients with metastatic disease without specific treatment remains limited, multiple new treatment options developed during the past 2 decades are now available for the treatment of metastatic disease. As a result, median survival has increased to nearly 30 months in the latest large randomized study, from approximately 6 months in the 1990s. This improvement in survival has been driven not by a single “magic bullet” but by the sequential deployment of a variety of chemotherapy and so-called targeted therapy agents. This latter class includes monoclonal antibodies to vascular endothelial growth factor (VEGF) (bevacizumab) and epidermal growth factor receptor (EGFR) (cetuximab and panitumumab), aflibercept, a recombinant fusion protein also directed against VEGF, and regorafenib, an active inhibitor of multiple tyrosine kinases. Finally, a small but substantial minority of patients with isolated sites of metastases may potentially be curable with surgery and liver-directed therapies.
The availability of multiple therapeutic agents for the treatment of metastatic colorectal cancer therefore requires a strategic approach to maximize patient benefit, in terms of both life expectancy and quality of life. When determining initial treatment, the first step is to evaluate whether the patient is potentially curable by a surgical resection of metastases either at the time of diagnosis or after conversion therapy. This approach will guide the choice and timing of chemotherapy. Treatments with the potential highest response rates and the greatest potential to downsize metastasis are the most appropriate for potentially curable patients. If the patient does not seem curable, treatment regimens that offer the longest progression-free survival (PFS) and overall survival (OS) and that maintain quality of life as long as possible are to be preferred. This review focuses on describing systemic approaches to the treatment of patients with metastatic colorectal cancer, with notes on the incorporation of liver-directed and primary resection modalities in the appropriate context.
Introduction
Metastatic colorectal cancer is an important contributor to the public health burden of cancer-related mortality. An estimated 136,830 people in the United States will be diagnosed with colorectal cancer in 2014. Approximately one-fifth of these patients will have distant metastatic disease at the time of presentation. The spread of primary colorectal cancer can occur by lymphatic and hematogenous dissemination, as well as by contiguous and transperitoneal routes. The presence of right upper quadrant pain, abdominal distension, early satiety, supraclavicular adenopathy, or periumbilical nodules usually signals advanced metastatic disease. However, given extensive staging and surveillance protocols, it is also common to identify metastatic disease based on imaging studies. The first site of hematogenous dissemination is usually the liver, followed by the lungs and bone. An exception is rectal cancer, which may metastasize initially to the lung because the inferior rectal vein drains into the inferior vena cava rather than into the portal venous system.
Although the prognosis for patients with metastatic disease without specific treatment remains limited, multiple new treatment options developed during the past 2 decades are now available for the treatment of metastatic disease. As a result, median survival has increased to nearly 30 months in the latest large randomized study, from approximately 6 months in the 1990s. This improvement in survival has been driven not by a single “magic bullet” but by the sequential deployment of a variety of chemotherapy and so-called targeted therapy agents. This latter class includes monoclonal antibodies to vascular endothelial growth factor (VEGF) (bevacizumab) and epidermal growth factor receptor (EGFR) (cetuximab and panitumumab), aflibercept, a recombinant fusion protein also directed against VEGF, and regorafenib, an active inhibitor of multiple tyrosine kinases. Finally, a small but substantial minority of patients with isolated sites of metastases may potentially be curable with surgery and liver-directed therapies.
The availability of multiple therapeutic agents for the treatment of metastatic colorectal cancer therefore requires a strategic approach to maximize patient benefit, in terms of both life expectancy and quality of life. When determining initial treatment, the first step is to evaluate whether the patient is potentially curable by a surgical resection of metastases either at the time of diagnosis or after conversion therapy. This approach will guide the choice and timing of chemotherapy. Treatments with the potential highest response rates and the greatest potential to downsize metastasis are the most appropriate for potentially curable patients. If the patient does not seem curable, treatment regimens that offer the longest progression-free survival (PFS) and overall survival (OS) and that maintain quality of life as long as possible are to be preferred. This review focuses on describing systemic approaches to the treatment of patients with metastatic colorectal cancer, with notes on the incorporation of liver-directed and primary resection modalities in the appropriate context.
Systemic regimens for metastatic colorectal cancer
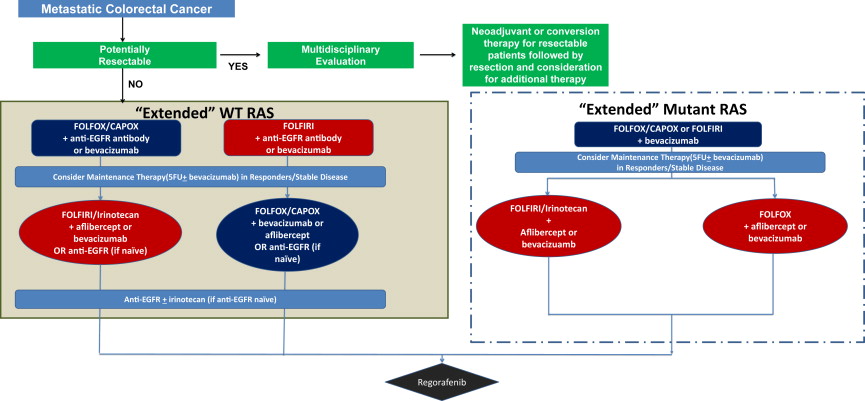
In patients with unresectable metastatic colorectal cancer, who comprise most cases, systemic treatment is focused on tumor control, which is occasionally symptom-directed (palliative) and not curative. The treatment goals are to increase life expectancy while maintaining quality of life for as long as possible. In this context, the model of distinct lines of chemotherapy is being abandoned in favor of a continuum-of-care approach similar to that taken in other chronic illnesses. Using currently available data, the authors propose an algorithm for treatment selection based on emerging clinical and molecular data ( Fig. 1 ).
The 3 active conventional chemotherapy agents for metastatic colorectal cancer are fluoropyrimidines (including intravenous 5-fluorouracil or its oral prodrug equivalent, capecitabine), irinotecan, and oxaliplatin ( Table 1 ). Patients clearly benefit from access to all active agents. A variety of targeted therapy agents are also available ( Table 2 ), which are incorporated into conventional chemotherapy regimens at various time points across the continuum of care.
| Category | Mechanism | Major Adverse Effects |
|---|---|---|
| Fluoropyrimidines (5-fluorouracil/capecitabine) | Thymidylate synthase inhibitors; the accumulated deoxyuridine monophosphate misincorporates into DNA, resulting in inhibition of DNA synthesis and function | Myelosuppression Mucositis and/or diarrhea Hand-foot syndrome |
| Irinotecan | Converted by carboxylesterase to SN38, which prevents the relegation of DNA and further results in double-strand DNA breaks and cellular death | Diarrhea Myelosuppression Alopecia |
| Oxaliplatin | A third-generation platinum compound, covalently binding to DNA, which results in inhibition of DNA synthesis and transcription |
|
| Category | Mechanism | Major Side Effects |
|---|---|---|
| VEGF inhibitors (bevacizumab, aflibercept) | Bevacizumab: a recombinant humanized monoclonal antibody to VEGF-A receptors | Hypertension Bleeding Gastrointestinal perforation Arterial thromboembolism (including stroke and myocardial infarction) |
| Aflibercept: a VEGF receptor decoy fusion protein consisting of extracellular domain components of VEGF-R1 and VEGF-R2 fused with the Fc region of IgG1 | ||
| Anti-EGFR antibodies (cetuximab, panitumumab) | Monoclonal antibodies against EGFR | Infusion reactions Hypomagnesemia Pruritus/dry skin Pulmonary toxicity Diarrhea |
| Kinase inhibitor (regorafenib) | A small-molecule inhibitor of multiple cell-signaling kinases | Hand-foot syndrome Fatigue Diarrhea Hypertension |
Initial Treatment: The Chemotherapy Backbone
Data from head-to-head comparisons suggest that outcomes with first-line oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX) and irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) are similar. Studies have evaluated the substitution of the oral fluoropyrimidine capecitabine for intravenous 5-fluorouracil, with either irinotecan (CAPIRI) or oxaliplatin (CAPOX). CAPIRI has been associated with excessive toxicity, and is not recommended. Data also suggest that CAPOX has similar antitumor efficacy but potentially more toxicity, especially thrombocytopenia, hand-foot syndrome, and diarrhea, but may be considered in patients unable to receive ambulatory infusional therapy. Irinotecan and oxaliplatin (IROX) may be considered in a small proportion of patients who are intolerant of 5-fluorouracil.
The choice between initial FOLFIRI and FOLFOX or CAPOX should be based on expected treatment-related toxicity in the context of coexisting comorbidities for any given patients. For instance, a patient with long-standing diabetes mellitus or preexisting neuropathy may be recommended FOLFIRI rather than the neuropathy-inducing FOLFOX regimen. For patients who are not candidates for an intensive oxaliplatin-based or irinotecan-based regimen because of comorbidities, performance status, or personal preference, fluoropyrimidine therapy alone (with or without a targeted therapy agent) can be considered. In early studies, high rates of successful resection and favorable long-term survival rates for patients with initially unresectable liver metastases have been reported for a triplet regimen that combines all 3 classes of conventional chemotherapy (FOLFOXIRI), and this regimen may be considered in highly select patients until additional randomized data are available.
For patients receiving a FOLFIRI-like regimen after progression on FOLFOX, expected response rates are between 4% and 20%, and PFS of 2.5 to 7.1 months, respectively. On the other hand, studies of oxaliplatin-based therapy in patients failing an initial irinotecan-based regimen describe response rates around 10%, and median time to progression (TTP) of 4 to 5 months. The current standard approach to metastatic colorectal cancer includes either oxaliplatin-based therapy after progression on a FOLFIRI-like regimen, or irinotecan-based therapy after progression on a FOLFOX-like regimen; most clinicians, including the authors, prefer an oxaliplatin-based regimen first, based on perceived higher response rate and slightly better toxicity profile, although neither of these perceptions has been substantially proved in head-to-head studies. FOLFIRI is preferred in patients with a history of adjuvant FOLFOX in the preceding 12 months.
The Chemotherapy Backbone: Bottom Line
- •
Either FOLFOX or FOLFIRI can be used in the first-line setting, depending on patient preferences and concerns about specific toxicities.
- •
Single-agent fluoropyrimidine may be used in settings where patients are unable or unwilling to receive combination therapy.
- •
FOLFOXIRI may be considered in highly select patients in whom a high response rate and aggressive approach are warranted.
- •
Targeted agents should be added to all chemotherapy backbones when possible.
Incorporating Anti–Vascular Endothelial Growth Factor Agents
The anti-VEGF monoclonal antibody, bevacizumab, does not have significant single-agent activity in metastatic colorectal cancer. However, multiple clinical trials have shown that it adds benefits to first-line fluoropyrimidine-, oxaliplatin- and irinotecan-based regimens given across the continuum of care in patients with metastatic disease. In the randomized TREE-2 trial, for instance, adding bevacizumab to oxaliplatin and 5-fluorouracil-containing regimens in previously untreated patients resulted in a median OS of 23.7 months versus 18.2 months for the combined non–bevacizumab-treated groups. The addition of bevacizumab to oxaliplatin-based regimen in previously treated patients with metastatic colorectal cancer (with 5-fluorouracil or irinotecan) also led to improved PFS (7.3 vs 4.7 months) and median OS (12.9 vs 10.8 months) compared with FOLFOX alone in the ECOG 3200 trial conducted in the second-line setting. Thus, the accepted consensus in the management of metastatic colorectal cancer is to add bevacizumab to the chemotherapy backbone chosen for the individual patient for initial or subsequent treatments. Bevacizumab is associated with increased rates of grade 3 or 4 hypertension, bowel perforation, impaired wound healing, arterial thromboembolism, and bleeding events. Careful patient selection and monitoring for toxicity is important, as is timing of discontinuation of treatment if surgical intervention is to be considered. Given that the half-life of bevacizumab is approximately 3 weeks, the authors’ general recommendation is to hold bevacizumab for a period of 4 to 6 weeks before and after surgery to avoid postsurgical complications such as wound dehiscence.
Traditional teaching with conventional treatments has been to discontinue all classes of drugs when patients progress. It is unclear, however, whether a similar strategy should be adopted with biological agents, particularly those with antiangiogenic activity. The efficacy of continuing bevacizumab beyond progression was tested in the ML18147 phase III trial, which demonstrated a significant improvement in PFS (5.7 vs 4.1 months) and OS (11.2 vs 9.8 months). Bevacizumab-related adverse events were not increased in comparison with historical data of first-line bevacizumab treatment.
Aflibercept is a recombinant fusion protein that binds with higher affinity to VEGF-A than does bevacizumab in a cell-free system. In the United States, aflibercept is approved for use in combination with FOLFIRI for the treatment of patients with metastatic colorectal cancer that is resistant to or has progressed following an oxaliplatin-containing regimen, based on the placebo-controlled VELOUR trial. The median OS was significantly longer in patients treated with aflibercept (13.5 vs 12.1 months) as was median PFS (6.9 vs 4.7 months). Treatment benefit was similar regardless of prior bevacizumab exposure (about 30% of patients in the trial). However, there are no head-to-head trials comparing continuation of bevacizumab beyond progression with switching to aflibercept in this setting; hence, either strategy is considered appropriate.
Incorporating Anti–Epidermal Growth Factor Receptor Agents: Role of Precision Therapy
Anti-EGFR agents can improve outcomes in metastatic disease, both as single agents and in combination regimens. Biomarker analysis is critical to patient selection for therapy with an EGFR inhibitor, a form of so-called precision or personalized medicine whereby tumor mutations in individual specimens are used to select systemic therapy. Activating mutations in KRAS, which result in constitutive activation of the RAS-RAF-ERK pathway, lead to resistance to anti-EGFR therapy. In 2009, the American Society of Clinical Oncology recommended that all patients being considered for anti-EGFR therapy undergo KRAS mutation testing of their tumors, and that treatment with these agents be restricted to those with wild-type (WT) KRAS, defined as an absence of mutations in exon 2 of KRAS gene by qualitative real-time polymerase chain reaction. Emerging data suggest that resistance to anti-EGFR therapies can also be mediated by lower-frequency mutations in KRAS outside of exon 2 and in NRAS. In 2013, the PRIME study demonstrated that within the so-called classic WT KRAS population (without mutations in exon 2), patients with mutations in other KRAS exons (exons 3 and 4) or in NRAS (exons 2 and 3) did not benefit from the addition of panitumumab to FOLFOX. Of concern, such patients had a nonsignificantly worse PFS (7.3 vs 8.0 months, P = .33) and OS (hazard ratio 1.39, P = .12) with the addition of anti-EGFR therapy. Furthermore, the addition of panitumumab to chemotherapy increased OS significantly in patients with so-called extended WT RAS (no mutations in exons 2, 3, and 4 of KRAS and NRAS). Other analyses have confirmed these findings. The emerging consensus is that all patients with metastatic colorectal cancer should be tested for extended RAS mutations and that those with such mutations should not be recommended anti-EGFR therapy.
First-line cetuximab was explored in the CRYSTAL trial in previously untreated metastatic colorectal cancer; patients were randomly assigned to FOLFIRI with or without cetuximab. Among patients with WT KRAS, response rates were significantly higher in those who received cetuximab (57% vs 40%), as was median PFS and OS (23.5 vs 20 months). The EPIC trial randomly assigned 1298 oxaliplatin-refractory patients to irinotecan with or without cetuximab. PFS was significantly higher with combined therapy (4 vs 2.6 months), as were rates of objective response (16% vs 4%) and overall disease control (61% vs 46%). Increasing data also support the efficacy of first-, second-, and third-line panitumumab in combination with oxaliplatin-based or irinotecan-based regimens in patients with WT RAS tumors. Cetuximab and panitumumab appear to have comparable efficacy when used as single agents for salvage therapy in patients with chemotherapy-refractory metastatic colorectal cancer, and when used for initial or subsequent therapy for metastatic colorectal cancer in conjunction with an irinotecan-based chemotherapy regimen. The choice of anti-EGFR agent largely depends on provider comfort with specific agents, concerns regarding infusional reactions, and logistics (cetuximab is a weekly regimen whereas panitumumab is every other week).
Although response rates in individual studies were higher with the addition of cetuximab to chemotherapy than by adding bevacizumab in patients with WT KRAS status, the median survival benefit is similar. Where there is likelihood of converting patients to resectable metastatic disease, the improved response rates support using anti-EGFR therapy in the first-line setting. Dual antibody therapy targeting both VEGF and EGFR has been tested and found to lead to worsened outcomes, and is therefore not recommended.
Among patients with WT RAS, an important issue is whether to add bevacizumab or anti-EGFR therapy to the chemotherapy backbone as initial therapy. Two initial trials with small sample sizes suggested benefit for an “anti-EGFR first” approach. However, definitive results from the largest such study, C80405, a US Intergroup study, were presented in 2014 and showed no survival difference for either cetuximab or bevacizumab when combined with a chemotherapy backbone in the initial treatment setting (29.9 vs 29 months, P = .34). Therefore, either antibody can be used in the initial therapy setting, with choice driven again by toxicity, patient preference, and logistics. It should be noted that C80405 did include patients who may have had extended RAS mutations (only classic KRAS mutants were excluded); results of this subgroup analysis may alter the final conclusions in the future.
Regorafenib
Regorafenib targets a variety of kinases implicated in angiogenic and tumor growth-promoting pathways. Its activity in refractory metastatic colorectal cancer was demonstrated in the CORRECT trial. Patients who had progressed after multiple standard therapies assigned to regorafenib had a modest though statistically significant improvement in median OS (6.4 vs 5 months), and PFS (1.9 months vs 1.7 months). At present, regorafenib is reserved for patients whose cancers have progressed on the other standard chemotherapeutic and targeted therapy agents.
Targeted Therapy: Bottom Line
- •
All chemotherapy backbones in initial and subsequent settings should be accompanied by one targeted agent, unless specific contraindications exist.
- •
Anti-EGFR approaches should be used only in patients with extended RAS WT tumors.
- •
Bevacizumab or anti-EGFR therapy may be used in the initial setting in such extended RAS WT patients.
- •
Bevacizumab may be continued beyond progression with a change in chemotherapy backbone.
- •
Dual antibody therapy should be avoided.
Maintenance regimens
Approximately 75% of patients discontinue first-line chemotherapy in trials for reasons other than progressive disease, and face the question of whether to consider maintenance chemotherapy or take a chemotherapy break. The OPTIMOX trials showed that oxaliplatin can be safely stopped after 6 cycles in a FOLFOX regimen, and that complete discontinuation of chemotherapy had a negative impact on PFS compared with maintenance therapy with 5-fluorouracil. Results from CAIRO-3 showed that maintenance therapy with bevacizumab plus capecitabine after 6 cycles of CAPOX plus bevacizumab was associated with a significant longer PFS (8.5 vs 4.1 months). Decisions regarding maintenance therapy versus treatment breaks must also take into account patient preferences and cost.
Potentially curable advanced colorectal cancer
The liver is the first site of hematogenous dissemination in most patients with colorectal cancer. Approximately 10% of patients can live past 5 years even with metastatic disease. The definition of resectable metastatic disease is evolving, and there is not an accepted standard: even bilobar metastases and extrahepatic disease are no longer considered contraindications. Decision-making in this setting requires multidisciplinary collaboration: a practical approach requires that patients should be medically fit for surgery, existing liver disease should be resectable with adequate liver remnant, and extrahepatic disease should be controlled. Surgical resection is the preferred treatment in patients with oligometastatic disease primarily in the liver, with 5-year survival rates of approximately 50% to 60% in patients with favorable prognostic features. Approximately one-fifth of such patients survive beyond 10 years in some series, and this applies also to nonhepatic disease in certain series. When the primary tumor site is controlled and the metastatic disease is limited in lungs without extrapulmonary location (except for resectable or resected hepatic lesion), resection of isolated pulmonary metastases can increase survival rates up to 40% at 5 years.
Select patients with initially unresectable liver metastases may become eligible for resection if the response to chemotherapy is sufficient. This approach has been termed conversion therapy to distinguish it from neoadjuvant therapy. Conversion therapy allows 12% to 33% of initially unresectable or borderline resectable metastases to become eligible for metastasectomy. Five-year survival rates average 30% to 35%, which is substantially better than expected with chemotherapy alone.
A regimen with a high likelihood of objective response is typically chosen because of the strong correlation with subsequent resection rates. However, the choice of regimen is not well established. The triplet chemotherapy regimen FOLFOXIRI with bevacizumab was associated with significantly higher response rates (65% vs 53%) and PFS (median 12.2 vs 9 months) in comparison with FOLFIRI plus bevacizumab in the phase III TRIBE trial. However, FOLFOXIRI did not result in a significantly higher secondary complete (R0) liver resection rate (15% vs 12%), and was associated with greater adverse effects. Combination of anti-EGFR inhibitor with either irinotecan-based or oxaliplatin-based regimens has shown modestly improved resection rates in patients with WT KRAS status. The German multicenter randomized phase II trial (CELM study), by using FOLFOX plus cetuximab or FOLFIRI plus cetuximab, showed 62% tumor response in all patients with 70% in WT KRAS patients, but no OS and PFS improvement. A promising recent phase II study reported a surgical R0 resection conversion rate of approximately 70% (14 of 20 patients) with FOLFOX with dose-escalating cetuximab in initially unresectable patients with WT KRAS status. Hepatic intra-arterial chemotherapy either alone or in addition to systemic therapy also has the potential to downstage hepatic metastases. However, there are no randomized trials comparing hepatic pumps with contemporary systemic chemotherapy alone, and this approach is not currently widely used in the United States.
For patients with initially resectable liver metastases, a common sequence (particularly for patients with synchronous metastatic disease) is initial systemic chemotherapy, mainly to obtain prognostic information, treat potentially disseminated micrometastases as early as possible, evaluate for emerging additional metastases, and test the chemosensitivity of the tumor. Upfront surgery is an appropriate option for patients with metachronous presentation of hepatic metastases. The European Organization for Research and Treatment of Cancer Intergroup trial 40983 enrolled 364 resectable patients with up to 4 metastases without prior exposure to oxaliplatin who were randomly assigned to liver resection with or without perioperative FOLFOX chemotherapy. Initial chemotherapy improved patient selection for hepatic resection. The postoperative complication rate was significantly higher in the chemotherapy group (25% vs 16%). However, the postoperative mortality was not higher than surgery alone (1 vs 2 deaths). In the latest update, at a median follow-up of 8.5 years, there was a nonstatistically significant trend in 5-year PFS favoring chemotherapy (38% vs 33%), but 5-year OS was not significantly better in the chemotherapy group (51% vs 48%). A recent retrospective study suggested that neoadjuvant therapy only benefits high-risk patients. In patients with more than 2 risk factors, those who received neoadjuvant chemotherapy had improved median survival (38.9 vs 28.4 months). By contrast, for low-risk patients, survival outcomes were similar with or without neoadjuvant chemotherapy, median survival (60.0 vs 60.0 months) and 5-year OS (64% vs 57%, P >.05).
Liver metastases recur in up to 80% of patients after liver resection, with approximately half being confined to the liver. In these patients, provided that the aforementioned resectability criteria are fulfilled, repeat liver resection is safe and can lead to survival rates that are equivalent to those reported for first hepatectomy. It is therefore important to monitor patients carefully to detect hepatic recurrence at a resectable stage. Only limited evidence is available regarding the optimal follow-up strategy after liver resection for metastatic colorectal cancer. The following surveillance strategy for patients with metastatic disease rendered disease-free is reasonable: carcinoembryonic antigen, liver function tests, and computed tomography scan of the chest, abdomen, and pelvis every 3 to 6 months for 2 years, then every 6 to 12 months for up to 5 years.
Other Liver-Directed Treatments
Several other regional therapies, including local tumor ablation, regional hepatic intra-arterial chemotherapy or chemoembolization, and stereotactic body radiation therapy, are options for patients with liver-isolated colorectal cancer metastases who are not candidates for surgery. These therapies are often incorporated with initial hepatic resection or used as alternatives in patients who are not medically fit enough to undergo surgical resection. Although these methods can provide excellent local control, long-term survival outcomes are not well studied.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree