Adrenergic symptoms
Palpitations (5 %)
Diaphoresis (30 %)
Anxiety (12 %)
Tremors (12 %)
Hyperphagia/obesity (44 %)
Neuroglycopenic symptoms
Visual changes/diplopia (42 %)
Confusion/altered mental status (67 %)
Amnesia (8 %)
Abnormal behavior (16 %)
Lethargy/weakness/fatigue (28 %)
Headache (7 %)
Seizures (16 %)
Coma
These symptoms present in the fasting state and can become evident following physical exertion. They normally occur at plasma glucose levels less than 50 mg/dL, and central nervous system (CNS) symptoms mainly present at plasma glucose levels less than 45 mg/dL [5]. Some patients may not manifest significant symptoms even with significant hypoglycemia, which is termed hypoglycemic unawareness, when the CNS adapts to frequent episodes of hypoglycemia [17].
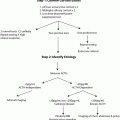
Diagnosis
Patients often present with bizarre symptoms not unique to hypoglycemia. These patients are often falsely diagnosed with psychiatric or neurologic disorders, resulting in a delay in diagnosis of insulinoma . The mean duration to diagnosis has been reported to range from several months to even more than several decades. One study reported a median of 18 months (range 1–240 months) to diagnosis [18]. In another study, the mean duration was noted to be 3.8 years ranging from 1 h (in a newborn) to 34 years [19].
The classic diagnosis is achieved by the presence of Whipple’s triad:
1.
Fasting hypoglycemia (plasma glucose < 50 mg/dL)
2.
Neuroglycopenic symptoms
3.
Relief of symptoms after administration of glucose
The normal negative feedback mechanism is altered in patients with insulinoma. Insulin production is not dependent on the blood glucose level and continues during periods of low blood glucose levels. The biochemical diagnosis is obtained by measuring elevated insulin in the presence of hypoglycemia. The gold standard biochemical diagnosis consists of the 72-h fast requiring hospitalization with measurement of plasma glucose, insulin, C-peptide, and proinsulin. It is able to detect 99 % of insulinomas [20].
The patient is admitted for a monitored fast. During the fasting period, the patient is allowed to drink calorie-free fluids and is encouraged to ambulate. Blood glucose is initially measured every 6 h [21, 22]. Once the plasma glucose level reaches less than 60 mg/dL, measurements are taken every 1–2 h [21, 22]. When the glucose level reaches 40–45 mg/dL with the presence of symptoms, blood is drawn to measure glucose, insulin, C-peptide, β-hydroxybutyrate, and sulfonylurea [21, 22]. The fast is then terminated. The presence of low glucose (≤ 40 mg/dL), high insulin (≥ 6 μU/L), elevated C-peptide (≥ 200 pmol/L), and absence of sulfonylurea in toxicology exam are all defining features of insulinoma [23]. Level of proinsulin ≥ 5 pmol/L and β-hydroxybutyrate levels ≤ 2.7 mmol/L are also measurements that can guide diagnosis [23].
Proinsulin is a precursor to endogenous insulin, which undergoes enzymatic cleavage into insulin and C-peptide [22]. With surreptitious insulin use or oral hypoglycemia, the proinsulin level would be normal or decreased, allowing the test to distinguish other conditions causing fasting hypoglycemia [24]. Factitious hypoglycemia due to exogenous insulin would also result in low C-peptide levels [24].
After completion of the test, some centers administer 1 mg of intravenous glucagon and take measurements of plasma glucose levels after 10, 20, and 30 min to measure the response of beta-hydroxybutyrate and glucose, which increases the sensitivity of the test [25]. Insulin is an antiketogenic and glycogenic agent; therefore, low levels of beta-hydroxybutyrate ≤ 2.7 mmol/L at time of hypoglycemia or increase in plasma glucose > 25 mg/dL within 30 min of the glucagon bolus can be used as surrogate markers of hyperinsulinemia [22].
In more recent years, a 48-h fast has been suggested to replace the traditional 72-h fast with the diagnosis of insulinoma achieved in 90–97 % of patients [26].
Outpatient fast protocols have also been developed [25]. Patients are instructed to start the fast at home overnight, and the office visit is timed for when the usual hypoglycemic symptoms occur. If the glucose level does not drop during the visit, the patient is admitted for continued fast as an inpatient.
Differential Diagnosis
Hypoglycemia can be caused by diabetes mellitus, sepsis, drugs, critical illness, renal/hepatic failure, and hypocortisolism. Hypoglycemia in healthy-appearing patients can also be caused by noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS), factitious hypoglycemia, strenuous exercise, and ketotic hypoglycemia. NIPHS is an endogenous hyperinsulinism not caused by a functional tumor [25]. Diffuse islet cell hyperplasia/nesidioblastosis can also cause exaggerated insulin response to glucose ingestion. These conditions present with postprandial hypoglycemia 1–4 h after meals, with similar symptoms as insulinomas , but with different timing and pathology [12]. Autoimmune hypoglycemia which consists of elevated insulin due to anti-insulin antibodies or anti-insulin receptor antibodies can also be part of the differential diagnosis [27].
Localization
Preoperative localization of insulinomas is important in determining the surgical approach and the amount of resection needed as well as in decreasing the operation time. The failure rate of preoperative localization is reported to be around 10–27 % [28]. Some suggest that preoperative localization is not necessary because they are successfully localized intraoperatively, but the need for imaging still exists to rule out metastatic disease and to decide on the extent and type operation [29, 30].
Several methods of noninvasive imaging are utilized with varying sensitivities and advantages. Transabdominal ultrasound is an easily accessible, cost-efficient modality without exposure to radiation, but it is highly operator dependent and limited by the patient’s body habitus. Obesity, which is a common characteristic of insulinoma patients, decreases its effectiveness. Resolution is also often limited by the presence of bowel gas overlying the pancreas [25]. However, when used appropriately, it can be useful in identifying the size, location, and relationship of the tumor to surrounding structures. Sensitivity ranges from 9 to 67 % and therefore can be used as a preliminary study, but most patients undergo other imaging modalities [8].
On dual-phase computed tomography (CT), insulinomas often appear as a hypervascular, well-defined, round, homogenous mass with enhancement during the arterial phase. Atypical appearances can occur with hypoattenuation, cystic mass, and calcification, which is discrete, nodular, and more common in malignant tumors [31, 32]. Dynamic CT is especially useful due to the small size of most insulinomas. The location of the tumor, its relationship to surrounding structures, as well as lymphadenopathy and presence of metastasis can all be evaluated. CTs have the advantage of being easily interpreted by the operating surgeon. With the recent advances in CT technology, sensitivities have increased [33]. The latest multiphase helical CTs can localize 50–80 % of insulinomas [24]. Multidetector CTs can visualize 94.4 % of insulinomas (Fig. 1) [34].
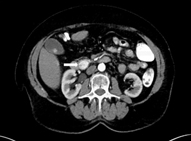

Fig. 1
CT of the abdomen. A 74-year-old female patient with a 1.8-cm insulinoma in the head of the pancreas inferior to ampulla of Vater
Magnetic resonance imagings (MRIs) have become a superior modality for small insulinomas. They are safe and noninvasive, and have the ability to detect metastasis. Insulinomas have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
Sensitivity is reported to be 40–95 % [33]. Diffusion-weighted MRI offers even better imaging. MRIs also have the advantage of being more sensitive than CT for liver metastasis (Fig. 2) [24].
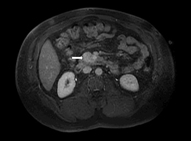

Fig. 2
MRI of the abdomen. A 49-year-old male patient with a 3.6-cm insulinoma in the uncinate process
Despite the common use of CT and MRI for insulinoma localization, there is a significant number of insulinomas that are undetectable by these noninvasive means. The subsequent use of some invasive techniques may allow localization of these tumors.
Endoscopic ultrasonography (EUS) is an invasive procedure that requires sedation and is operator dependent but has high detection rates of 86.6–92.3 % [35]. Sensitivity varies from 37 to 94 % depending on the location of the lesion [8, 36–39]. Sensitivity is higher in the head of the pancreas and lower for the tail and splenic hilum area. Using a high-frequency transducer (7.5–10 MHz), EUS is able to detect lesions as small as 5 mm. Insulinomas have a characteristic appearance on EUS: They are homogeneously hypoechoic, round and have distinct margins. In young, female patients with low body mass index (BMI), tumors can be isoechoic and easily missed by EUS [40]. EUS-guided fine-needle aspiration (FNA) can also be performed when the appearance of the tumor is unusual to confirm the diagnosis, raising the sensitivity of the technique. Fritscher-Ravens et al. used a 22-gauge needle to obtain adequate tissue in two to three passes to diagnose and differentiate neuroendocrine tumors from each other as well as from other malignancies (Fig. 3) [41].
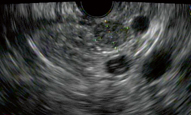

Fig. 3
EUS showing 11-mm insulinoma in the body of pancreas appearing as a round, hypoechoic lesion with well-defined borders. FNA confirmed pancreatic endocrine tumor and patient underwent a laparoscopic enucleation of the tumor
Angiography and arterial stimulation venous sampling was previously one of the major localization methods with sensitivity of 87.5–100 %. However, due to its invasive and technically demanding nature, and the development of other imaging modalities, it is no longer the first-line modality [42, 43].
Transhepatic portal-venous sampling (THPVS) requires percutaneous and transhepatic catheterization of a branch of portal vein. The catheter is then advanced into small veins that drain the pancreas. It requires intra-arterial and intravenous catheterization. This technique had a localization rate of 64–100 % [8]. However, it has now been replaced by intra-arterial calcium stimulation with hepatic venous sampling [44, 45].
Intra-arterial calcium stimulation with hepatic venous sampling provides additional information. The tumor can be localized by its hormonal function allowing development of an accurate surgical approach and avoidance of reoperation [46]. The accuracy of this technique is around 94–100 % (17431724) and sensitivity of 77–100 % [8, 42, 43, 47–49].
The tumor appears as a well-defined, round/oval vascular blush, with increased vascularity [25]. It is visualized in the early arterial phase and continues through the venous phase. The mechanism involves increased calcium in vessels causing degranulation of cells, releasing insulin into the portal venous system, leading to a detectable increase in insulin in venous samples obtained from the hepatic vein [50]. Selective catheterization of splenic (body/tail), gastroduodenal (anterosuperior head), superior mesenteric (posteroinferior head), and proper hepatic (hepatic mets) arteries is performed with simultaneous venous catheterization of the right hepatic vein for obtaining blood samples [25]. Sequentially 0.025 mEq/kg of calcium gluconate are injected, and blood samples are obtained to measure insulin levels from right and left hepatic veins prior to and 30, 60, and 120 s after calcium infusion [51]. A twofold increase in insulin concentration from baseline localizes the insulinoma to its respective region. It is important to account for aberrant anatomy because localization depends on normal anatomy of the pancreatic vasculature.
Unlike other pancreatic neuroendocrine tumors , somatostatin receptor scintigraphy is not helpful for insulinomas. The test is positive in only 46 % of benign insulinomas. Not all insulinoma cells express somatostatin receptor type 2, which explains the low yield of this test. Malignant insulinomas can have a higher rate of positive yield [23].
Management
Surgical resection is the primary treatment modality for all localized tumors. Surgery has a high success rate with cure obtained in more than 90 % of patients [19, 44].
Patients are generally admitted 1 day prior to surgery for monitoring of blood glucose. After midnight, the patient is maintained on a 10 % dextrose infusion. Some centers promote the use of diazoxide preoperatively for glucose control. Precise intraoperative glucose monitoring is highly important. The level of blood glucose is kept above 40–50 mg/dL using dextrose boluses if necessary [25]. The open approach involves a bilateral subcostal incision and the abdomen is first examined for any signs of metastases [25]. An extended Kocher maneuver is performed to access the head, and uncinate or medial mobilization of the spleen and distal pancreas is performed if tumor location is distal. Manual palpation has a sensitivity of 75–95 % in locating the tumor [52]. Intraoperative ultrasound (IOUS) is also used with sensitivity of 80–100 % (Fig. 4) [53, 54].
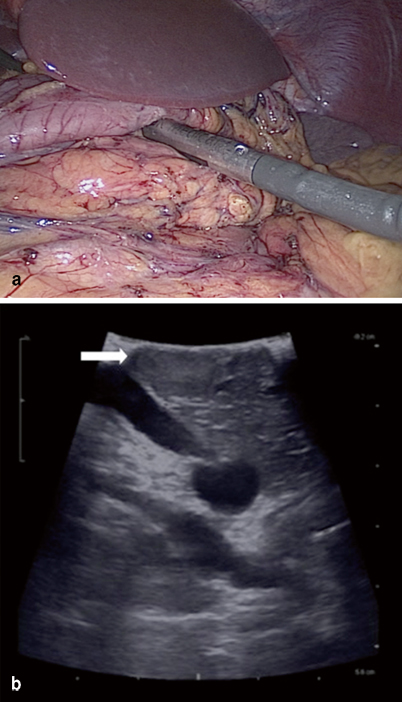

Fig. 4
a Intraoperative ultrasound can be performed to localize insulinoma. b Ultrasound showing 1.5-cm tumor located in body of pancreas
The combination of palpation and IOUS allows 83–98 % of tumors to be detected [24]. IOUS is useful for defining the anatomy—the relationship of the tumor to pancreatic and bile ducts as well as the vasculature. Benign, solitary lesions are amenable to enucleation , with complete excision of the capsule to prevent local recurrence [22]. Usually, a clear dissection plane exists between the tumor and the pancreatic parenchyma. Even when the tumor is located close to the common bile duct (CBD) or pancreatic duct, enucleation can be performed in most cases because the tumor usually does not invade or constrict the ducts [25]. Enucleation and partial pancreatectomy preserve the pancreatic parenchyma and reduce the risk of exocrine/endocrine insufficiency [55]. Distal pancreatectomy may be required, if the tumor is located deep in the tail, and in some rare cases, pancreatoduodenectomy may be necessary, if the tumor is located deep in the head [18]. Also extended resections may be necessary for multiple tumors, large tumors > 4 cm, tumors that are not well encapsulated, or those suspicious for malignancy—tumors that are hard, infiltrating, or puckering, or have evidence of pancreatic duct dilatation, indicating duct involvement and probable malignancy. In cases of suspected or proven malignancy, peripancreatic lymphadenectomy should be performed to verify the extent of disease [25]. The open approach may be indicated for MEN-1-associated insulinomas, because multifocal disease and presence of other tumors must be considered. These tumors may require subtotal pancreatectomy in addition to enucleation (Fig. 5) [56, 57].
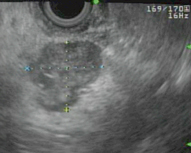

Fig. 5
Patient with MEN-1 and multifocal insulinoma. EUS showing well-defined 2.1-cm irregular mass in the pancreatic body, numerous subcentimeter lesions throughout the rest of the body of the pancreas, and single subcentimeter lesion in the tail of the pancreas. Patient underwent a laparoscopic spleen-preserving distal pancreatectomy and pathology confirmed multifocal insulinoma
Once the tumor is removed, blood glucose levels may rapidly rise to 120–140 mg/dL. Glucose is administered intravenously for an additional 24 h postoperatively. High glucose levels may persist for several days to weeks, and the patient may require doses of insulin administration. Blood glucose is measured frequently during this period. Normalization of glucose levels is observed usually within 2–3 weeks after surgery [25].
In cases where the tumor could not be localized intraoperatively, blind distal pancreatectomy was performed historically. However, this approach has been abandoned due to a low success rate. Since there is equal distribution of insulinomas throughout the pancreas, a blind distal pancreatectomy would only resolve half of the tumors, those located in the body and tail. With the current multimodality preoperative imaging options as well as improved intraoperative ultrasound, blind resections are no longer recommended [58].
Laparoscopic resection of insulinomas was started in 1996 by Gagner et al. [59] . Others soon followed and there are multiple publications regarding the technique, which is becoming more and more popular for benign, small insulinomas located in the body or tail of the pancreas. Distal pancreatectomy can be performed with electrocautery or other endoscopic sealing device [25]. Preoperative localization is even more important in these cases because intraoperative palpation is not performed. Laparoscopic IOUS can now identify > 85 % of tumors. Laparoscopic resection has a success rate of 70–100 % and is valued for its shorter duration of stay and faster recovery of patients [24, 60, 61].
Postoperative complications include pancreatic leaks that can present as fistulas or intra-abdominal collections, which are self-limited and most can be treated conservatively. Fistula rates have been reported to be around 15–43 % [24, 62–64].
Abscesses requiring percutaneous drainage consist about 4–6 % [24]. Delayed gastric emptying occurs in 4–8 % of patients and is mainly managed with medical treatment. Other possible complications are hemorrhage, pseudocyst, diabetes, and pancreatitis [25]. Up to 40–50 % can present with these various complications [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree