BACKGROUND
Before the advent of insulin in 1922, pregnancy in a woman who had diabetes was uncommon and perinatal mortality rates ranged from 40% to 60%. While the discovery of insulin was revolutionary, White reported in 1928 that 25% of pregnancies in women with diabetes resulted in stillbirth.1 Even as late as 1980, women were still being advised to avoid pregnancy because of the persisting poor obstetric history in 3050% of women with diabetes.2 In the intervening years, perinatal mortality rates have finally improved, most likely as a consequence of increasing sophistication of obstetric and neonatal care, widespread use of cesarean delivery and the advent of capillary home glucose monitoring. Despite these advances, perinatal mortality and congenital malformation rates remain threeto fivefold higher than in the general maternity population.
The relevance of hyperglycemia to maternal and perinatal outcomes is now clearly established. Poor control in early pregnancy contributes to an increased risk of spontaneous abortion and fetal malformations, and in late pregnancy to an increased risk of preeclampsia, hydramnios, macrosomia, operative delivery, and stillbirth. Unfortunately patients still present late in gestation with poor glycemic control. Optimizing insulin delivery remains demanding for both the patient and clinician.
This chapter will review the current approaches to optimizing insulin delivery in diabetic pregnancy. The primary focus will be on Type 1 diabetes and data from randomized trials, but the literature is limited. The key question is whether any treatment strategy is safe and superior to another in improving maternal/fetal outcome.
PHYSIOLOGY
An understanding of “normal” carbohydrate metabolism of pregnancy is necessary to the achievement of normoglycemia.2 The pregnant woman with Type 1 diabetes requires sufficient insulin to compensate for increasing caloric needs, increasing adiposity, decreasing exercise, and increasing anti-insulin hormones (Table 10.1). There is also increased degradation of insulin in pregnancy and a reduced rate of glucose disposal. The normal pancreas can adapt to these factors by increasing the insulin secretory capacity. As the mechanism of disease in Type 1 diabetes involves destruction of beta (insulinproducing) islet cells, the need for insulin increases, and failure to increase insulin doses accordingly results in hyperglycemia.
PATHOPHYSIOLOGY
In vivo and in vitro studies have clearly shown that hyperglycemia is toxic to the embryo. Clinically, the picture is that of fasting and postprandial hyperglycemia. The presumption is that either sustained or intermittent hyperglycemia stimulates fetal insulin secretion with consequent increased fetal growth. Reports of macrosomia despite excellent metabolic control may possibly be explained by unrecognized glucose spikes, especially postprandially. Placental transfer of insulin complexed with IgGs has been associated with fetal macrosomia in mothers with normal glycemic control during gestation. However, in a prospective study of 42 women with gestational diabetes mellitus (GDM) who failed diet control, there was no difference in antiinsulin antibody levels, gestational age at birth or birthweight between women randomized to either insulin lispro (n = 19) or regular insulin (n = 23). The area under the glucose curve during a test meal was significantly lower in the lispro group.3
Table 10.1 Metabolic alterations in Type 1 diabetic pregnancy.
| Early pregnancy |
| • Loss of glucose and gluconeogenic substrates |
| • Blood glucose control is more unstable than usual |
| • Nocturnal hypoglycemia is common |
| • Insulin requirements can fall by 1020% at end of the first trimester, possibly due to a fall in progesterone or change in human chorionic gonadotropin (hCG) or thyroid hormone and/or decreased nutrient intake due to pregnancyrelated nausea |
| Mid pregnancy |
| • Change to a lipid-based energy economy |
| • Insulin requirements begin to increase |
| • Progressive increase in insulin resistance at the level of the skeletal muscle and fat cells |
| Late pregnancy |
| • Increased production of antiinsulin hormones |
| • Anti-insulin hormones include human placental lactogen, prolactin, estrogen, maternal production of cortisol |
| • Insulin requirements increase to twice or more the prepregnancy total daily dosage |
| • Insulin requirements may plateau or even decrease towards term |
Glucose levels during pregnancy and in relation to adverse outcome
Much interest has centered on fetal macrosomia defined as birthweight greater than 4000g or at or above the 90th centile for gestational age (large for gestational age). Two US studies reported on diabetic patients using the White classification, which categorizes patients by age of onset, diabetes duration, and presence of vascular complications rather than mechanisms of disease; most of these patients were likely to have had Type 1 diabetes. In the first study, Landon et al4 showed that among 75 women with White Class B through D, those with a mean capillary blood glucose greater than 6 versus less than 6 mmol/L (108 mg/dL) during the second and third trimester had significantly increased rates of fetal macrosomia (28% vs 17%, respectively; p < 0.05) and neonatal respiratory distress (21.1% vs 2.3%; p < 0.01). In the other study, Jovanovic et al5 reported that 52 insulindependent women with diabetes (White class B through F), who maintained “normal” blood glucose levels from 12 weeks of gestation (fasting 3.0-3.6mmol/L [54-65 mg/dl], mean glucose 4.4-4.8mmol/L [79-87mg/dL], and postprandial <7.7mmol/L [139mg/dL]) had infants with a mean birthweight of 2910 g and none was above the 75th centile or developed respiratory distress. More recently, Nielsen et al6 described a dose-dependent association between HbA1c levels greater than 7% in the first trimester and risk of adverse outcome among 573 Danish women with Type 1 diabetes.
PREPRANDIAL VERSUS POSTPRANDIAL CAPILLARY GLUCOSE MONITORING
Traditional approaches have measured capillary glucose before meals. Prolonged postprandial hyperglycemia, however, is the primary manifestation of hypoinsulinemia and its relevance to outcome has come under increasing scrutiny. Several studies have compared preand postprandial glucose measurements with neonatal macrosomia, but the results are conflicting. Among three studies which controlled for confounders, two reported that postprandial, but not fasting, glucose was predictive of birthweight percentile7 and macrosomia.8 In contrast, the third study found that fasting, but not postprandial, glucose was significantly associated with relative birthweight.9
Two randomized trials compared fasting and preprandial (target 3.3-5.9mmol/L [60-106mg/dL]) with postprandial glucose monitoring (target < 7.8mmol/L [<140mg/dlL]). De Veciana et al10 randomized 66 insulinrequiring Latina women with gestational diabetes mellitus at less than 30 weeks of gestation and found that the postprandial group had a lower mean HbA1c predelivery (8.1% vs 6.5%; p < 0.001), lower rates of cesarean section due to cephalopelvic disproportion (12% vs 36%; p ≤ 0.04), and large for gestational age offspring less frequently (12% vs 42%; p = 0.01) than the preprandial group. Manderson et al11 randomized 61 Type 1 diabetic women at 16 weeks and reported a significantly reduced incidence of preeclampsia (55% vs 30%; p < 0.001), greater success in achieving glycemic control targets (55% vs 30%; p < 0.001) and smaller neonatal triceps skinfold thickness (4.5 ± 0.9 vs 5.1 ± 1.3; p = 0.05) in those whose insulin dose was based on postprandial glucose measurements.
GLUCOSE AND HbA1c VALUES IN NORMAL PREGNANCY
In pregnant women without diabetes Parretti et al12 reported a mean capillary glucose of 3.11 mmol/L (56.0 mg/dL) between 28 and 38 weeks of gestation, with the mean peak postprandial response occurring at 1 hour and never exceeding 5.84 mmol/L (105 mg/dL). There was a significant association between fetal abdominal circumference and 1hour postprandial g lucose (p < 0.0003).
Neilsen et al showed that healthy pregnant women have significantly lower HbA1c concentrations than nonpregnant women.13 In addition, the upper limit of normal of HbA1c decreased from 6.3% in early pregnancy to 5.6% by late pregnancy, which is of clinical importance when defi ning a reference range for HbA1c during pregnancy.13
RECOMMENDATIONS FOR GLUCOSE MONITORING AND TARGETS
Current guidelines are outlined in Table 10.2. Glucose targets vary somewhat depending on the expert body recommendation.14,15 Women should be supported and encouraged to monitor their blood glucose regularly. The evidence suggests that additional benefit is to be gained from a combination of preand postprandial monitoring. The 1hour postprandial time point is monitoring is advised fasting and 1h postprandially (and for insulin treated, at bedtime) optimal as it corresponds most closely to the postprandial glucose peak and has been related to fetal outcome. In Type 1 diabetes, blood glucose should be measured eight times daily (before and after each meal, at bedtime and in the middle of the night). In Type 2 diabetes/ GDM, the frequency of monitoring is to some extent dictated by the treatment strategy, but more frequent measurements identify more frequent hyperglycemia, leading to a higher insulin usage and a lower incidence of macrosomia. Although interpretation of HbA1c results is made difficult by methodologic variation and the fall in HbA1c during normal pregnancy, it provides an important index of periconceptional hyperglycemia. While the UK National Institute for Health and Clinical Evidence (NICE) guidelines do not recommend serial HbA1c measurements during pregnancy, the authors believe that knowledge of periodic HbA1c readings, with reference to a nonpregnant reference range, may be reassuring to the patient, and if raised, provides the clinician with an additional pointer to the problematic pregnancy.
Table 10.2 Recommended targets for capillary whole blood glucose during pregnancy.
| American Diabetes Association (ADA)14 * | |
| • Preprandial glucose | ≤5.8 mmol/L (105 mg/dL) |
| • 1 h Postprandial glucose | ≤7.8 mmol/L (140 mg/dL) |
| or | |
| • 2 h Postprandial glucose | ≤6.7mmol/L (120mg/dL) |
| National Institute for Health and Clinical Excellence (NICE)(2008)15** | |
| • Fasting | 3.5-5.9 mmol/L (63-106 mg/dL) |
| • 1-h postprandial | <7.8 mmol/L (140 mg/dL) |
* ADA does not distinguish between different type of diabetes
** NICE states that targets should be individualized;
INTENSIVE INSULIN REGIMES
In most countries intensive insulin regimes usually comprise an MDI of shortacting insulin before meals and intermediateacting insulin at bedtime (often given with a pen device). Some advocate the need to give the basal isophane insulin more than once daily,5 and there may be justification for this approach with the increasing use of insulin analogs, and if the gap between meals is greater than 3 hours. In the US, a greater proportion of subjects who have Type 1 diabetes use continuous subcutaneous insulin infusion (CSII) outside of pregnancy and this method is then continued in the maternity setting (see below). Some patients, particularly those with Type 2 diabetes, use a twicedaily fixed mixture insulin regime prepregnancy, but are often changed to an MDI regime for the duration of pregnancy.
The advantage of MDI over traditional regimes is that blood glucose levels can be tightly controlled by frequent selfregulated adjustment of dose, necessary because of the dynamic insulin requirements of pregnancy. To some extent this has evolved with changes of diabetes care. The Diabetes Control and Complications Trial (DCCT) (1990s) was pivotal in leading to the acceptance that MDI regimes prevented longterm consequences of diabetes, but data supporting such regimes in pregnancy are limited. Post hoc analysis of the DCCT trial of women who subsequently became pregnant on the different regimes did demonstrate better outcome for multiple injections than more conventional regimes.16
Nachum et al17 reported a randomized trial comparing outcomes in women with diabetes who received insulin four times daily (138 GDM and 58 pregestational) with twice daily (136 GDM, 60 pregestational). Glycemic control was better with the four times daily regime: in GDM women mean blood glucose concentrations decreased by 0.19 mmol/L (3.5 mg/dL) (95% CI 0.13–0.25) and HbA1c by 0.3% (95% CI 0.2–0.4%); in women with pregestational diabetes; corresponding values for mean blood glucose were 0.44 mmol/L (8.0 mg/dL) (95% CI 0.28– 0.60) and HbA1c 0.5% (0.2–0.8%). In women with GDM, the four times daily regime resulted in lower rates of neonatal hyperbilirubinemia (RR 0.51, 95% CI 0.29–0.91) and hypoglycemia (RR 0.12, 95% CI 0.02–0.97). The relative risk of neonatal hypoglycemia in women with pregestational diabetes was 0.17 (95% CI 0.04–0.74).
Insulin treatment in gestational diabetes mellitus and Type 2 diabetes
Prospective studies of insulin treatment for GDM have generally shown a reduction in the likelihood of macrosomia.14 In a small study of women with GDM randomized to receive only porcine regular insulin before each meal or a morning dose of Neutral Protamine Hagedorn (NPH) insulin, those receiving premeal short-acting insulin had infants with lower birthweight (3.079 ± 0.722 vs 3.943 ± 0.492 kg) and a lower rate of macrosomia.18 As the mechanisms of disease are similar for both GDM and Type 2 disease (i.e. increased insulin resistance resulting in beta cell exhaustion), the management of insulinrequiring GDM and Type 2 diabetes is identical.
Comment on insulin regimes
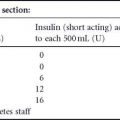
Most patients are now using an MDI regime although data supporting such regimes in pregnancy are limited. Patients must be encouraged to check their blood glucose regularly and record the results in a form that permits recognition of glycemic patterns and which allows gradual prospective insulin adjustments (Table 10.3).
INSULIN ANALOGS
There has been increasing interest in the role of insulin analogs outside of pregnancy. Their main advantages are fewer episodes of hypoglycemia, a reduction in postprandial glucose excursions, and an improvement in overall glycemic control and patient satisfaction. A Cochrane review, however, concluded that evidence for their benefit over regular and NPH insulin was minimal. Although physiologically attractive in pregnancy, data here are even more limited and have largely been provided by clinical experience. Relevant issues include potential teratogenicity, mitogenicity, and interaction with the insulin-like growth factor (IGF-1) receptor.
Table 10.3 Insulinadjustment examples.
| • If 1-h postprandial glucose is >7.8mmol/L(140mg/dL) add 2 U of regular insulin to dose impacting that meal the following day |
| • If 1-h postprandial glucose is <3.3mmol/L (59mg/dL), decrease dose of regular insulin impacting that meal by 2 U following day or increase interval between insulin injection and meal |
| • If fasting glucose is >5.5mmol/L (99mg/dL), add 2U to 2200-h intermediate-acting insulin, but check 0300-h blood glucose level before 2200h insulin dose is increased. A 0300h plasma glucose <3mmol/L (54mg/dL) would point to unrecognized nocturnal hypoglycemia as an explanation for the elevated fasting glucose. In this case, decrease the 2200-h intermediate-acting insulin by 2U while carefully monitoring the fasting glucose level |
| • If fasting glucose is <3.3mmol/L (59mg/dL), decrease 2200-h long-acting insulin by 2U |
| • If glucose is elevated during late afternoon, increase morning dose of intermediateacting insulin following morning (if taking intermediateacting insulin) or increase noon dose of shortacting insulin (if on prandial insulin) |
| • If glucose is low mid-morning and mid-afternoon, reduce morning dose of shortacting insulin (frequently to resolve both problems) |
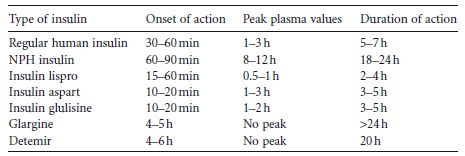
Three shortacting human insulin analogs and two longacting human analogs are currently commercially available (Table 10.4).
Randomized trials
Several small randomized trials of insulin lispro in pregnancy have been published. Among women with GDM3,19 or Type 1 diabetes treated from week 15,20 those for whom insulin lispro analogs were added to basal NPH insulin had fewer hypoglycemic episodes3 and lower 1hour postprandial glucose levels,19,20 but no difference in overall blood glucose levels or HbA1c3,20 compared with subjects receiving regular human insulin. Birthweight, macrosomia, cesarean delivery, neonatal hypoglycemia, and antiinsulin antibody levels3 were similar between the two groups.3,20 Cypryk et al21 reportedpregnancy outcome among 25 women with Type 1 diabetes treated with lispro compared with 46 controls (who were maintained on the same type of insulin as prepregnancy); maternal and fetal outcomes in the two groups were similar.
Two studies have been reported using insulin aspart.22,23 Pettitt et al22 randomized 27 women with GDM to aspart or regular human insulin before meals, and found safety and effectiveness were comparable in the two groups. In the largest randomized controlled trial to date,23 157 pregnant women with Type 1 diabetes were randomized to insulin aspart and 165 to regular human insulin. There were fewer episodes of nocturnal hypoglycemia (RR 0.48, 95% CI 0.20–1.14; p = 0.10) and severe hypoglycemia (RR 0.72, 95% CI 0.36–1.46; p = 0.36) in the aspart group, although neither of these achieved statistical significance. Prandial increments were significantly lower in the aspart group in the first and third trimester. No differences were observed in 24hour mean glucose profiles or HbA1c. Perinatal outcomes were similar between the two groups. It was concluded that aspart was at least as safe as human insulin regarding perinatal outcome.23
Table 10.4 Pharmacokinetic properties of regular human insulin and rapidacting analogs.