Introduction
Infections are a common cause of hospitalization for patients with opioid use disorders (OUDs). In 2012, there were 530,000 OUD-related hospitalizations with a total estimated healthcare cost of $15 billion [ ], approximately 6.500 of those admissions were related to infections, doubled from the previous decade, with an associated total cost of $700 million [ ]. This is an underestimate in the context of the growing opioid crisis over the past decade in the United States. There is likely underreporting of infections related to OUD; chronic infections acquired via substance use are also not included in such estimates, such as Hepatitis C virus (HCV) or HIV infection and their downstream morbidities. Many receiving inpatient care for infectious issues do not have a diagnosis of OUD. Data from the 2016 National Survey on Drug Use and Health describe 11.8 million people age 12 and over as having misused opioids (IV or oral) in the past year with only 2.1 million of them having been diagnosed with OUD. This gap represents patients who are at high risk for developing OUD or potentially have undiagnosed disease.
For many patients with OUD, an inpatient hospital admission represents a “reachable moment” for life-saving interventions [ ]. First, the patient can be identified and diagnosed with OUD and can be initiated on medications for treatment of OUD (MOUD): methadone, buprenorphine, or extended-release naltrexone. Second, comorbid conditions such as psychiatric comorbidities can be identified. The hospital setting can be a venue where OUD is reframed as a chronic relapsing medical disorder for the patient and managed as such, in a person-centric manner. Integrating treatment in a multidisciplinary manner is essential. With MOUD-based therapy and integrated medical team-based care, treatment outcomes can be optimized with a reduction in interrupted care and hospital discharges against medical advice (AMA). The infectious disease (ID) physician can and should play an integral role in these missions.
Pathophysiology of infections related to OUD
The vast majority of infections related to OUD are bacterial complications related to needle-associated pathogen entry [ ]. Most of these infections are from the patient’s own skin flora at time of needle puncture during injection drug use (IDU), and consequently streptococcal species and Staphylococcus aureus are the most common [ ]. Site of injection informs the commensal flora involved; gram negative and anaerobic infections are more common in those who use groin access for injection [ ]. Practices of general hygiene and skin decontamination with alcohol swabbing prior to injection may reduce abscess formation [ ]. Although people who inject drugs (PWID) may think their drugs are contaminated [ ], it is uncommon for infections to result from contamination of drug supply or paraphernalia [ , ].
Drug preparation and injection techniques play a large role in the microbiology of injection-related infections. Water used as heroin solvent can contain environmental gram-negative organisms, especially if sourced from clearly nonsterile sites (i.e., toilet water). Needle licking predisposes to infections with oral streptococcal and anaerobic species. Such practices are performed with the misconception that licking can clean the needle and a desire to “not waste” residual heroin on the syringe [ ]. Acidification of opioids with nonsterile ascorbic acid sources such as citrus juices and fruit can be a source of Candidal exposure [ ].
Needle reuse dulls the bevel of the instrument and increases the likelihood of trauma and venous damage during injection, predisposing to a skin or soft tissue infection (SSTI). Needle and syringe sharing is the key mode of transmission for viral infections such as HIV and HCV. The original case-control studies for HCV risk in the United States showed a 49-fold higher risk of seropositivity in IDU [ ]. HIV and HBV both have an increased prevalence in PWID though the association is more nonspecific given transmission via sex. It is estimated that roughly 15%–20% of PWID are HIV positive globally but there are vast differences from country to country [ ]. Improper decontamination of reused paraphernalia is common, such as lack of awareness of need for bleach products, or decontamination of the needle portion and not the syringe. Clonal analysis of bacteremias caused by S. aureus in clustered drug networks suggests that it can be transmissible by drug paraphernalia as well [ ].
OUD not related to IDU (i.e., via smoking, snorting, or prescription pill use) technically should not have the same associated infectious risk factors, although any lifetime history of previous IDU could confer similar risk. Growing evidence supports epidemiological associations of oral opioid use with other conditions such as invasive pneumococcal disease [ ]. Transactional sex and survival sex in exchange for opioids can also elevate transmission of STIs including HBV and HIV.
Inpatient OUD screening in the infectious disease consultation
Screening for OUD
A cross-sectional evaluation performed in 2009 of a large inpatient urban center found a substance use disorder incidence of approximately 11% (excluding alcohol, tobacco, and patients already stabilized on MOUD) [ ]. The United States Preventative Services Task Force in 2008 found insufficient evidence for universal screening in the general medical setting for drug use. These guidelines are currently being revised to respond to the national OUD crisis, and other organizational bodies such as the National Institute for Drug Abuse (NIDA) recommend universal OUD screening [ ]. We recommend consideration of OUD screening for all ID consultations, especially in those that may be IDU related. Optimally, initial ID consultation will occur early so as to potentially link to MOUD treatment and improve inpatient outcomes.
Certain infections and conditions elevate the pretest probability of having OUD. As mentioned above, HCV, HIV, and HBV infections all increase the likelihood of IDU-associated OUD. Bloodstream infections without a clear source such as Staphylococcus bacteremia or Candidiasis merit further OUD screening. Other conditions related to bacteremia or contiguous spread such as infectious endocarditis, osteomyelitis, or endophthalmitis could be injection related. Numerous noninfectious risk factors for OUD also exist. For the patient with prescribed oral opioids, four behavior patterns have been associated with illicit use: early refills, intoxication with the prescribed drug, dose increase of the patient’s own volition, and oversedation [ ]. Relevant state Prescription Monitoring Program review can display patterns of medication seeking. Finally, patients with frank opioid withdrawal or intoxication can be misdiagnosed without clinical suspicion for OUD.
The NIDA Quick Screen is a single screening question for past year use of alcohol, tobacco, nonmedical prescription drug, and illegal drug use [ ]. It is meant for use by general medical practitioners and is an effective screen for OUD and other SUD. The doctor-patient discussion on screening is best prefaced by ensuring confidentiality and appropriate medical care. A positive screen reflexes to the NIDA-Modified Alcohol, Smoking, and Substance Involvement Screening Test ( NM-ASSIST ), which assesses for severity for a variety of illicit substances. The screening process is typically interpreted in tandem with a brief intervention (referred to as SBI) with a goal to frame a discussion and support deeper diagnostic evaluation. The discussion has been framed as the “5 As” of SBI: Ask, Advise, Assess, Assist, and Arrange . The provider begins by asking permission to discuss screening results, and then advises on drug use. Assessment regards the patient’s readiness to quit—both readiness to start MOUD and/or harm reduction services (e.g., naloxone proficiency, needle syringe exchange) should be queried. Subsequently assistance is given in making behavioral changes, and “arrange” refers to creating follow-up care. The 5A framework should be tailored to the patient interview—if screening and OUD diagnosis occur at the same time, the intervention portion can be tailored to a discussion on MOUD initiation.
Urine toxicology screening is best used as an additional data point with a given sensitivity and specificity to be interpreted in a clinical context. Most clinical drug screening is a urine immunoassay in which an antibody panel binds against drugs or metabolites. Its major drawback is cross-reactivity, which can be assay-dependent, and includes cross reactions with naloxone (false positive for oxycodone) and both fluoroquinolones and rifampicin (false positives for opiates) [ ]. Poppy seeds are known to have minute but detectable concentrations of morphine derivative that can trigger positivity. Confirmatory testing using liquid chromatography or mass spectrometry is typically not performed in clinical labs. Positivity duration depends on opioid type: codeine, heroin, and morphine can be detected for up to 2 days and methadone can be detected for up to 3 days using specific methadone assays. The general “opiate” immunoassay will not detect oxycodone, fentanyl, or methadone, although specific screens that will check for a broader panel of opioids and metabolites are now available.
Establishing the OUD diagnosis
For those that screen positive, a full assessment of severity is merited, with the goal of establishing whether OUD is present or not. If inpatient Addiction Medicine consultation is present, this role can be referred or shared. However, part of the movement for broader MOUD prescribing involves additional providers such as ID consultants to have competency with establishing a use disorder diagnosis.
Per the DSM-5 criteria depicted in Table 10.1 , an OUD constitutes a problematic pattern of opioid use resulting in clinically significant impairment. The three broad criteria categories are (1) loss of control, (2) adverse consequences, including health, legal, etc., and (3) physiology of tolerance demonstrated in the past 12 months.
|
Presence of two or more criteria is consistent with a diagnosis of OUD. Severity is determined by total number of criteria, though frequency and amount of opioid use is used in conjunction with diagnostic severity for a more holistic understanding of drug-related dysfunction.
There are a variety of diagnostic assessment tools to be used for establishing the diagnosis of OUD. The use of a particular assessment tool depends in part in clinical role (addiction specialist/psychiatrist vs. generalist; researcher vs. clinician) and whether there is motive for in-depth diagnosis of other substance use disorders. The SCID , or structured clinical interview for DSM-V, is the gold standard for in-depth psychiatric or research-based evaluation [ ]. It is a semistructured clinical interview based off of the DSM criteria. The Mini-International Neuropsychiatric Review, or MINI , is shorter and has been validated in relation to the SCID [ ]. All are designed to maximize diagnostic performance and interprovider reliability. For the targeted diagnosis and rapid treatment of OUD, direct use of the DSM-5 criteria serves an important role, especially in decentralized OUD treatment for nonaddiction specialty providers. The Rapid Opioid Dependence Screen (RODS ), developed by co-author S. Springer, is an eight question brief assessment tool validated against the MINI specifically for rapid diagnosis of OUD in incarcerated populations with HIV and could be used more broadly with subsequent scale validation [ , ].
Clarifying OUD for the infectious disease physician
Following diagnosis of OUD, there are two main goals: to detail the patient’s opioid use with particular attention paid to infection-related risk practices and to discuss initiation of MOUD. The clarification of OUD component can be woven into the framework of an ID consult history and physical and will guide the consultation.
Type, route, and frequency of opioid will greatly affect infection risk. Often patients will have a combination of different opioid use routes (e.g., injection, snorting, oral, and/or smoked) and types (e.g., both injection-based heroin/fentanyl and oral prescription illicit opioids). Site of injection can span from low-risk sites such as the antecubital fossa or hand to more central sites with higher commensal flora burden like the groin. Very high-risk injecting such as arterial or carotid (“shooting for big red”) injection has an elevated risk for aneurysm formation and life-threatening bleeding. Skin decontamination practices should be queried especially if the patient’s chief diagnosis is SSTI. Harm reduction requires hand hygiene and decontamination with proper equipment such as alcohol-based wipes. Skin-popping and muscle popping, which are subcutaneous and intramuscular injections, respectively, carry higher rates of abscess formation and spore forming bacterial infections. There is a wide spectrum of water sterility used among PWID. In ideal harm reduction settings, sterile water should be used, but sources including toilet water have been reported, often in the throes of withdrawal with limited alternative sources. This is fueled by misconceptions that the cooking of product will fully sterilize any infectious material. Paraphernalia sharing should be queried, with specific questions toward which pieces of equipment were shared and how—even if a syringe tip is replaced and a plunger is retained, residual body fluids could be reservoirs for HIV or HCV. Finally, there are a variety of real-world practices that PWID employ in attempts to sterilize paraphernalia. The most advisable form of decontamination is with undiluted household bleach retained for at least 2 min [ ]. Local availability of other harm reduction strategies such as needle and syringe exchange programs and overdose education and naloxone distribution will guide subsequent counseling.
Expanding on past substance use history is informative in anticipation of initiating MOUD treatment. A detailed history will include past use of MOUD, response, and information on relapse. History of overdose, hospitalization, and rehabilitation therapy is also relevant. The Prescription Monitoring Program database will provide historical data in regard to prescribed opioid use—methadone will not be reported here and, if prescribed, the relevant opioid treatment program (OTP) should be contacted. There is a significant heritability to substance use disorder that should be explored for family history. Social support and living situation contributes to psychosocial risk factors for use but also assistance networks for treatment. A history of legal issues regarding substances assesses adverse consequences of drug use.
Comorbid psychiatric disease can have a major impact on treatment outcomes if left undiagnosed or unmanaged. The introductory Patient Health Questionnaire (PHQ)-2 screen is a well-validated, brief screen for depression that can lead to further assessment of major depressive disorder with the PHQ-9 [ ]. Positive screening for suicidal or homicidal ideation requires psychiatric referral and stabilization. The NM-ASSIST, introduced above, can serve as a screen for other substance use disorders if performed to completion. Screening for chronic infectious diseases associated with IVDU such as HIV, HCV, and HBV should occur.
Withdrawal treatment with transition to medication maintenance
Opioid withdrawal treatment includes the patient-centered and evidence-based management of withdrawal symptoms using pharmacotherapy ideally with the use of opioid agonist therapy. Hospitalized patients with OUD may exhibit symptoms along the spectrum of acute intoxication to opioid withdrawal based on the timing of their opioid use. Goals of therapy for withdrawal treatment regard (1) humane care and relief of withdrawal symptoms, (2) forging of a therapeutic alliance, (3) retention in care, and (4) transitioning to maintenance use of MOUD (agonist vs. nonagonist therapy). Notably, there is no role of withdrawal (or the older stigmatizing terminology of “detoxification”) for achieving sustained abstinence from opioids [ ]. When a patient is in withdrawal, it is an ideal time to start an MOUD such as buprenorphine such that the withdrawal ceases, the patient remains in care, and is then able to be linked to outpatient care with a prescription upon release from the hospital.
Opioid withdrawal is a physiologic response to cessation of opioids after administration at dependence-inducing doses. Signs of withdrawal include insomnia, dysphoria, nausea/vomiting, lacrimation/rhinorrhea, pupillary dilation, yawning, diarrhea, tachycardia, and muscle aches. Temperature dysregulation and fever can occur. The physiology of opioid withdrawal is based on two main loci of neurotransmitter disruption: norepinephrine activity in the reticular activating system and dopamine activity in the mesolimbic pathway [ ]. Increased norepinephrine levels in withdrawal lead to symptoms of tachycardia, piloerection, myalgias, and irritability. The mesolimbic pathway, also colloquially understood as the “reward” pathway, has low levels of dopamine transmission in withdrawal, associated with dysphoria, craving, and depression. The Clinical Opioid Withdrawal Scale (COWS) has high sensitivity and interuser reliability and allows quantification of symptoms in the clinical setting [ ]. Pharmacologic treatments for withdrawal are based on targeting these specific pathways and are listed in Tables 10.2 .
| Agonist |
|
| Nonagonist |
|
Alpha-2 adrenergic agonists relieve the autonomic symptoms of opioid withdrawal. Both clonidine and lofexidine, the latter of which was FDA approved in the United States in 2018, are superior to placebo in treatment completion. Opioid agonist-based therapy treats both autonomic and dopaminergic (craving based) symptoms and is superior to alpha-2 adrenergic therapy for treatment completion [ , ]. These nonopioid agonist therapies are now used for adjunctive support or in institutional settings in which MOUD are not available.
Medications for OUD
Methadone
Methadone was FDA approved for OUD in the 1970s making it the oldest and most well-studied MOUD. Methadone is a weak opioid agonist, meaning it mimics the effects of opioids such as heroin and reduces withdrawal symptoms without causing euphoria [ ]. Because of its long half-life, daily methadone in the form of methadone maintenance treatment (MMT) can curb withdrawal symptoms for 24–36 h. Doses above 60 mg have consistently shown improved outcomes compared with lower doses [ ]. By preventing withdrawal, MMT reduces illicit drug use and subsequently reduces complications of nonprescription opioids: overdose, death, injection, and additional criminalized behaviors such as drug seeking [ ]. Methadone prevents the euphoria of heroin when used concurrently due to its effect on the opioid receptor, discouraging illicit drug use and promoting recovery.
In a clinical trial setting, methadone was significantly more effective than nonpharmacological interventions in its ability to retain patients in treatment and reduce heroin use [ ]. In a review of three randomized controlled trials (RCTs) comparing methadone to nonpharmacological approaches, methadone was unable to significantly reduce criminal activity or mortality, though subsequent large-scale cohort studies have established reductions in all cause and overdose mortality with treatment [ ]. Compared with buprenorphine, methadone is more effective at both low and flexible doses in retaining patients in treatment. At fixed medium and high doses, buprenorphine may be as effective as medium and high dose methadone, respectively, at retaining patients and suppressing illicit opioid use [ ]. However, fixed doses are impractical as part of routine care. MMT has also been shown to improve drug-related HIV risk behaviors, criminal behaviors, and mortality in addition to maternal and fetal outcomes.
Though methadone is a sufficient medication for treatment of inpatient opioid withdrawal, there can be dose and duration restrictions on inpatient prescribing which vary state by state, often making buprenorphine a more practical choice.
Clinicians and patients should appreciate the risks associated with MMT, including death, respiratory depression, and QT prolongation. For this reason, methadone dose should be slowly increased, and MMT should be avoided altogether in patients with a long QT. Patients receiving MMT should avoid additional respiratory suppressants including alcohol and benzodiazepines and medications that might increase the QT interval [ ]. Both methadone and buprenorphine are extensively metabolized by the CYP450 system (see Table 10.3 ).
| Medication | Buprenorphine | Methadone |
|---|---|---|
| NRTIs | None | ABC—decreased methadone levels. may need increased methadone dose |
| None | AZT—glucuronidation and renal clearance of AZT affected, monitor for zidovudine toxicity | |
| NNRTIs | EFV—PK effect (decreased), dose adjustments unlikely to be needed | EFV—decreased methadone levels, typically 30% dose increase needed |
| RPV—no adjustment | RPV—decreased methadone levels, may need increased methadone dose | |
| PIs | ATZ—Inc bup levels w/clinical correlate (oversedation, etc). Dose reduction or slower titration recommended | ATZ—mild increase in methadone levels, typically no dose change necessary |
| DRV/r—some PK effect (increased bup levels), dose adjustments unlikely to be needed | DRV/r—decreased methadone levels, may need increased methadone dose | |
| Integrase inhibitors | EVG/cobi—some PK effect (increased), dose adjustments unlikely to be needed | EVG—no effect w/cobicistat boosting, potential decrease of methadone levels with ritonavir, may need increased dose |
Buprenorphine
Buprenorphine is a semisynthetic derivative of thebaine with both partial agonist effect at the mu opioid receptor and antagonistic effect at kappa receptors [ ]. Due to these partial agonist properties, it exhibits a dose response curve with limited additional analgesia and euphoria, giving it a functional ceiling effect [ ]. Buprenorphine is highly potent and adherent to mu opioid receptors, and at moderate doses reduces cravings of illicit opioids through cross-tolerance and high-level receptor occupancy. This high-level occupancy, however, functions to displace other mu agonists which can lead to precipitated withdrawal.
Buprenorphine is ineffective via an oral route but is absorbed well via sublingual (i.e., transmucosal) and transdermal formulations. Naloxone has poor oral and sublingual bioavailability and as such plays no role in buprenorphine coformulated regimens (e.g., Suboxone) except to dissuade from snorting or injection of the medication. The four current formulations of buprenorphine are daily sublingual (tablet/film) or buccal (film), 6-month implantable (Probuphine), and monthly subcutaneous (Sublocade).
Drug allergies to buprenorphine are relatively rare, and it is advisable to educate patients on the difference between allergy and precipitated withdrawal symptoms. While the overdose potential is certainly lower than other full agonist opioids, it still exists—the risk of respiratory depression is increased when coadministered with benzodiazepines or alcohol [ ]. Buprenorphine can be used in situations of liver disease. Combination naloxone products are not recommended in severe hepatic impairment (Childs B or C); even the negligible amount of sublingual naloxone absorbed can become clinically significant given impaired hepatic metabolism and can cause precipitated withdrawal [ ]. Buprenorphine monotherapy can be used with dose reduction and caution for signs and symptoms of opioid toxicity. Hepatitis with fulminant hepatic failure has occurred in rare cases, typically in those with underlying liver disease such as underlying HBV and HCV. Unless there is known acute hepatitis, it is reasonable to prescribe and monitor closely without assessing LFTs prior to initiation [ ]. Notably, additional concurrent substance use disorders such as stimulant use are not contraindications to starting therapy. Buprenorphine is metabolized via the CYP3A4 hepatic system and as such is affected by other medications that either inhibit or induce this system as depicted in Tables 10.3 .
An abstinence period between last full mu opioid dose and initiation is required to avoid precipitated withdrawal. The abstinence period varies from approximately 12–16 h for short-acting opioids, 16–24 h for intermediate-acting opioids, and up to 48 h for high dose methadone dependence. In particular for the inpatient setting, it should be ensured that the patient is not receiving short-acting opioids for pain control. Typically at least mild withdrawal as noted by a COWS score of 5 or greater is sufficient to start treatment; however, some sources recommend a COWS score >12 as the goal prior to the first dose. An advantage to inpatient initiation of buprenorphine is the availability of a broad array of oral and IV medications to minimize withdrawal symptoms in the abstinence period; these should be liberally used. See Chart 10.1 for a depiction of initiating buprenorphine in the inpatient setting.
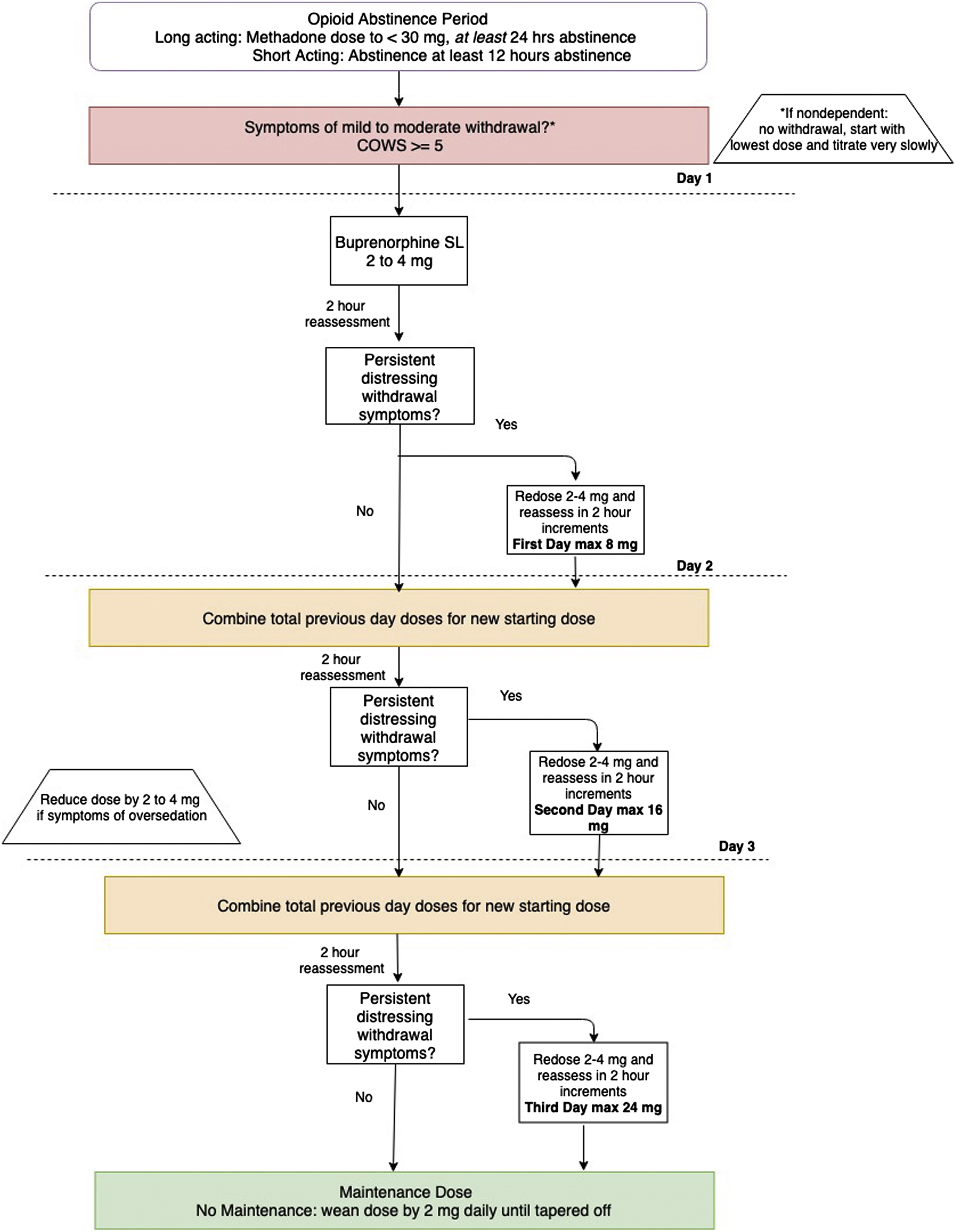
Patients should be informed not to swallow the sublingual medication and to allow for up to 10 min for it to fully dissolve before swallowing. The majority of patients stabilize on a maintenance dose of 8–16 mg, and the data for doses above 24 mg is currently limited [ ].
Two additional buprenorphine formulations of consideration are the subdermal implant, brand name Probuphine®, and extended-release buprenorphine, brand name Sublocade®. The implantable formulation deploys a steady state of 8 mg or less of buprenorphine over the course of 6 months. The extended-release formulation medication is administered monthly (300 and 100 mg doses) and designed for patients who have already been stabilized on at least 7 days of transmucosal buprenorphine at a dose of at least 8 mg [ ]. The inpatient setting may be an ideal time for transition to subdermal implantation or the extended-release buprenorphine formulation. Some patients will still require typically small doses of additional transmucosal agonist therapy to achieve resolution of cravings.
There is a subset of patients, particularly those with concurrent chronic pain, for which a period of opioid abstinence prior to induction might be infeasible. Transdermal buprenorphine (trade name Butrans®) appears to produce a more gradual exposure of opioid receptors to drug and is not associated with precipitated withdrawal when short-acting opioids are added. Regimens with initial transdermal buprenorphine with subsequent maintenance transition to sublingual dosing have been successfully reported and should be considered for those with chronic pain [ ].
Extended-release naltrexone (XR-NTX)
Naltrexone was created in the 1960s and first FDA approved for opioid blockade in 1984 as an oral formulation. The extended-release formulation (XR-NTX) was formulated much later and ultimately FDA approved in 2010 for maintenance treatment of OUD. Naltrexone is a competitive mu opioid receptor antagonist with no agonist properties; as such, it requires no waiver or regulatory requirements for inpatient or outpatient prescribing. The oral formulation is noneffective for OUD with no reduction in illicit opioid use relative to placebo, largely secondary to poor adherence [ ]. The extended-release formulation is a suspension of naltrexone embedded in biodegradable microsphere polymers meant for IM injection—dosing is 380 mg given every 4 weeks. Naltrexone is metabolized in the kidneys and liver (though not CYP450 mediated) and is excreted through the urine [ ].
Despite initial concerns of drug-related hepatitis, clinically significant elevations of transaminases with XR-NTX appear to be relatively uncommon, and advanced cirrhosis (Child-Pugh Class C) is the only true hepatic contraindication [ ]. Given its clearance via the kidneys, caution is advised when initiating in patients with moderate to severe renal impairment. The intramuscular route of entry is contraindicated in patients with severe coagulopathy. Naltrexone has not been studied thoroughly in pregnancy and hence its use is not recommended.
An appreciable period of opioid abstinence is required prior to XR-NTX initiation to prevent precipitated withdrawal. This is typically 7 days after last opioid use. This can be difficult in the outpatient setting, but can be manageable in the inpatient setting. There are some situations where this can be difficult, however, such as during surgical care requiring opioid analgesia. Patients should be counseled on the unique medication profile of naltrexone. The long abstinence initiation period might also be a barrier for some patients. Shared decision-making must be employed when selecting the ideal MOUD.
As naltrexone is not metabolized by the CYP450 system, there are relatively few drug-drug interactions. Injection site soreness is the most common side effect. More involved reactions such as hematoma, abscess, and cellulitis have been reported. At times it can be difficult to assess whether a patient has been adequately abstinent in order to initiate naltrexone. A naloxone challenge test can be considered prior to naltrexone initiation to avoid prolonged and unnecessary withdrawal. Though it can be useful, a negative naloxone challenge does not fully exclude in all cases that the patient will experience precipitated withdrawal [ ].
For patients who arrive to the hospital with withdrawal symptoms and opt for agonist-assisted withdrawal but naltrexone-based maintenance, timing can be a significant barrier. With up to 7 days of agonist-assisted withdrawal needed plus seven additional days of abstinence, this could add up to 14 days of inpatient hospitalization. In these cases, rapid naltrexone induction can be considered. The agonist-based withdrawal portion of therapy is shortened by administering single dose buprenorphine on arrival to sate craving and symptoms, followed by oral naltrexone the next day with nonopioid medications such as clonidine to ease the transition. This regimen increased recruitment onto XR-NTX and the likelihood to receive a second injection at week 5 (50% vs. 26.9%) when compared with full buprenorphine-based agonist withdrawal therapy followed by abstinence and XR-NTX in a clinical trial [ ].
Additional MOUD initiation considerations and therapy choice
One of the most impactful interventions for a patient admitted with OUD is initiation of FDA-approved MOUD. The main roles of inpatient initiation of MOUD are (1) agonist-based therapy for withdrawal treatment with taper (if per patient preference or in case of lack of outpatient MOUD), (2) withdrawal treatment with initiation of nonagonist-based therapy (i.e., XR-NTX) prior to discharge, or (3) continued agonist therapy as an outpatient. Of these options, continued outpatient MOUD therapy has the clearest and broadest benefit.
Choosing between MOUD therapies is based on a variety of factors including comorbidities, availability, medication profile, and patient preference. The patient’s motivation for long-term treatment is essential to this decision. Other considerations are future site of outpatient treatment, be it OTP versus office-based treatment; side effects or drug interactions; and the overall decision for agonist versus nonagonist-based therapy.
There is a preponderance of evidence for agonist-based therapy (i.e., methadone, buprenorphine) for treatment of OUD in order to achieve retention in treatment, reduction in illicit opioid use, and mortality reduction [ , ]. The benefits extend to numerous ID-related outcomes as well such as improved retention in ART, improved CD4, and viral suppression for HIV [ , , ]. Agonist therapies have been linked to increased retention in care for the HCV cascade and reductions of newly acquired HCV in PWID [ ]. There is currently no guideline recommendation to endorse one MOUD over the other. Both buprenorphine and methadone have a similar evidenced mortality benefit when used at adequate doses [ ]. Methadone in particular can only be prescribed and dispensed at a federally regulated OTP upon discharge. There are particular benefits for either continuing or initiating MOUD during the inpatient stay as well. Antagonist therapy, i.e., XR-NTX, is relatively newer and is associated with reduction in return to illicit opioid use and in treatment retention [ ]. XR-NTX has also been found to improve HIV viral suppression in persons living with HIV (PLWH) released from prison or jail with comorbid OUD [ ]. Additionally two large RCTs comparing buprenorphine to XR-NTX found similar opioid abstinence and retention outcomes when persons could successfully be inducted on XR-NTX [ , ].
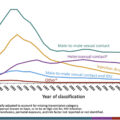
Patients are often motivated to start pharmacotherapy when they are hospitalized and dealing with the direct sequelae of their use disorder. In a survey of 29 hospitalized patients with IDU-related endocarditis who were offered an addiction psychiatry consultation, the majority (62%) accepted and initiated buprenorphine or methadone during the hospital admission [ ]. The inpatient setting has been validated as a feasible setting of MOUD as well. In a trial with randomization between inpatient buprenorphine administrations versus tapered withdrawal treatment with referral to an OTP, retention rates on buprenorphine at the 6-month mark were 16.7% versus 3% [ ]. The data show that MOUD is not a panacea for OUD that is a chronic relapsing disease, but given the known mortality of continued use, MOUD plays an important role. As it currently stands, less than a quarter of patients with opioid-related hospitalizations are offered MOUD upon discharge [ ]. This is suboptimal, and the disparity is particularly vast in patients with high morbidity infectious diseases such as infective endocarditis [ ]. It is poorly understood that inpatient providers without a DEA-waiver can prescribe agonist therapies such as buprenorphine and methadone. The Drug Addiction Treatment Act of 2000 was created as an exception to Controlled Substance Act to permit FDA Schedule III, IV, and V medications for OUD treatment to be prescribed as an outpatient outside OTPs. Buprenorphine of note is a schedule III drug. Outpatient prescribing does require a waiver to be obtained for buprenorphine, and, in contrast, XR-NTX requires no waiver for inpatient or outpatient prescribing. Federal law states that inpatient providers are exempt from waiver requirements for maintenance or withdrawal treatment if the patient is admitted for reasons that are not directly related to withdrawal (administering or dispensing of narcotic drugs, 21 CFR § 1306.07). In a significant amount of cases, these patients are hospitalized for infectious complications and the law allows for ID physicians to potentially be said prescribers.
Inpatient infections
More detail on SSTIs ( Chapter 8 ), Hepatitis C (Chapter 5), Hepatitis B ( Chapter 6 ), HIV ( Chapter 4 ), and endocarditis ( Chapter 9 ) is located elsewhere in this text.
Pulmonary infections
Older epidemiologic studies found that PWID had a 10-fold higher rate of pneumonia than the general population, independent of HIV status [ ]. A recent nested case control showed that opioid use itself (as measured from prescription refills) was associated with a 67% increase in invasive pneumococcal disease [ ]. These factors are likely from a combination of associated conditions, such as concurrent smoking affecting ciliary function and other potential substance use disorders such as crack-cocaine, together with an increased susceptibility for aspiration [ ]. Noninfectious insults can also cause underlying lung disease in heroin users such as emphysema, impairments in diffusion, and pulmonary hypertension at least partially from adulterants such as cotton and filler pieces depositing into lung tissue [ ]. PWID with community-acquired pneumonia are at higher risk for multiple complications such as parapneumonic effusion, empyema, and bacteremia [ ]. Notably, the syndrome of septic pulmonary emboli from endocarditis must be ruled out given significant change in management. IDU in particular is an independent risk factor for both latent TB infection (LTBI) and TB disease in low and high prevalence areas. Concurrent social determinants of health such as homelessness or incarceration contribute to the disparity, together with the increased prevalence of HIV. Notably the tuberculin skin test cutoff for LTBI treatment in PWID is 10 mm given the higher pretest probability in this population. One should consider drug-drug interactions for patients needing active tuberculosis treatment, as rifampin agents will have drug interactions with MOUD such as methadone and buprenorphine.
Bloodstream infections
Bloodstream infections result from either direct injection of bacteria from shared or dirty drug paraphernalia or inoculation of commensal flora. Invasive spread from a local SSTI may or may not also be apparent. It is key to have high suspicion and diagnostic rigor for infective endocarditis which would change medical medically management and potentially involve surgery. One cohort of 180 PWID hospitalized with bacteremia found that 74 patients, or 41%, had IE [ ]. The most common organisms involved are S. aureus , including community-acquired MRSA, and streptococcus. Injection drug users have higher rates of nasal S. aureus carriage than the general population. A cohort of urban poor in San Francisco had a carriage rate of 22.8%, and 12% of all assessed had community-acquired MRSA carriage. Other injection risk behaviors inform the microbiology involved. Use of contaminated water predisposes to environmental gram negatives including Pseudomonas aeruginosa , Sphingomonas , and Burkholderia species. Needle licking can introduce oral streptococcal species and anaerobes including Eikenella corrodens. Injection site in the lower half of the body such as groin injection will increase the likelihood of gram-negative involvement. Candidemia is relatively common among PWID, and a similar obligation exists to exclude IE. In addition, patients should be screened for visual symptoms and have an ophthalmologic examination to assess for chorioretinitis. Acidification of the injected drug to increase solubility with fruit or fruit juice is an established risk factor for Candida [ ]. Bacteremia with spore-forming bacteria such as Bacillus cereus or Clostridial spp. can occur in the presence or absence or an associated SSTI [ ]. Less common considerations include other gram-positive skin flora such as Corynebacteria spp. and coagulase negative Staphylococcus . Cotton fever is a self-limited febrile illness that occurs after heroin injection with reused cotton fiber. Typically fever occurs about 30 min after injecting, with leukocytosis and appearance of sepsis but with negative blood cultures. It results from a process of reheating and extracting used cotton filters, a process known as “shooting the cotton” [ ]. The pathophysiology is of presumed colonization of the cotton filter by Enterobacter agglomerans with subsequent endotoxin release.
Osteoarticular infections
Hematogenous seeding or local extension from SSTI can result in osteoarticular infections in PWID. Site of disease is informed by vasculature for hematogenous seeding and site of injection for local extension, and in some cases, both. Batson’s venous plexus is the network of veins draining visceral organs and extremities along the spine that, by virtue of their location and valveless nature, predispose to infective spread to the vertebrae. PWID are more likely to have cervical vertebral involvement in particular [ ]. Septic arthritis is more common on left-sided joints than the right, likely secondary to more right-handed persons injecting. As with other syndromes, ruling out infective endocarditis as a metastatic event is paramount. Providers should have a high index of suspicion for osteoarticular infections in drug use, as fever can be absent in up to one-third of cases, particularly in vertebral osteomyelitis with diskitis [ ]. Pyogenic organisms are the most common, with S. aureus being the most common followed by pyogenic streptococcal species (A, G). Anaerobes, particularly Eikenella corrodens , can cause what has been termed “needle lickers osteomyelitis” in patients with the relevant exposure. Tuberculosis should be considered in undifferentiated vertebral osteomyelitis and diskitis. Rare cases of atypical mycobacteria and molds such as Aspergillus have been reported to cause osteomyelitis as well. Candida can cause a particular constellation of costochondral lesions, folliculitis, and chorioretinitis in people injecting brown heroin [ ]. Infective spinal disease without source control or adequate treatment can result in contiguous spread and development of conditions such as psoas abscesses or spinal epidural abscesses. In the case of associated neurologic symptoms, urgent neurosurgical intervention is needed. Diagnosis should involve sampling of the affected site and bone biopsy, with 4–6 weeks of culture-directed antimicrobial therapy. Providers should be cognizant of acute pain management in this population as underlying opioid dependence may require higher doses of acute opioids to relieve patient symptoms and carry through with standard of care such as lying flat for MRIs or invasive procedures.
Endophthalmitis
Endophthalmitis is a potentially vision-threatening complication of intravenous drug use. Hematogenous spread, termed endogenous endophthalmitis, results in seeding to the highly vascular choroid plexus which can then progress through the retina and into the vitreous chamber. The aqueous chamber can be involved as well in severe cases. The bacteremia or fungemia associated with this dissemination is typically transient, and the majority of cases have negative blood cultures and are without fever; a vitreal tap with culture has a better yield for organism isolation [ ]. As such, providers should have a high degree of clinical suspicion in patients who use IV drugs with acute unilateral visual complaints. Common symptoms are eye pain, decrease in visual acuity, and visualization of floaters. Candida has long been known to be associated with endophthalmitis in patients with intravenous heroin and buprenorphine use. The incidence is likely increasing as the opioid crisis in the United States worsens [ ]. Treatment depends on the clinical syndrome and level of involvement of eye structures, as well as issues of ocular penetration. For those with solely chorioretinitis, azole-based therapy can be used based on susceptibility. Systemic amphotericin B with or without flucytosine is employed in azole-resistant isolates. Notably, because of suboptimal penetration into the retina and deeper vitreous, there is insufficient data for echinocandin use in chorioretinitis and it is not recommended in vitritis. If there is sight-threatening macular involvement or an element of vitritis, intravitreal antifungals (typically voriconazole or amphotericin B) are recommended in conjunction with systemic antifungals [ ]. In patients with nonresolving or worsening vitritis, there should be consideration in conjunction with ophthalmology of vitrectomy, of which there is growing evidence for improved outcomes with early intervention [ ]. Duration of therapy is at least 4–6 weeks or until resolution on ophthalmologic exam. Aspergillus is a less common cause of fungal endophthalmitis but can also be seen in PWID. Endogenous bacterial endophthalmitis is rare but has a distinctly rapid and site-threatening natural history. S. aureus is the most common organism, followed by B. cereus , the latter of which is likely introduced via contamination of drug product or paraphernalia. Progression from inoculation via transient bacteremia to eye pain with decreased visual acuity to vision loss can occur over the course of hours. Mainstay of therapy is culture-targeted intravitreous antibiotic; the role of adjunctive systemic antibiotics is a matter of debate but could potentially be useful.
Mycotic aneurysm and septic thrombophlebitis
Repeated suboptimal vascular access and endothelial damage from injection can cause sclerosis, fibrosis, thrombus formation, and aneurysms [ ]. The incidence of venous thromboembolism (VTE) in PWID is higher than the general population; a cross-sectional analysis of people with OUD in the United Kingdom reported an annual incidence of 3.2% and a lifetime prevalence of 14% [ ]. Septic thrombophlebitis should be suspected in clinical situations with bacteremia and underlying VTE. The femoral vein is the most common site of disease. S. aureus is the most common etiologic organism, followed by strep, with candida and gram-negative bacilli being identified less commonly. There is no clear prospective data to guide therapy—patients are often treated for 4–6 weeks with at least partial courses of parenteral antibiotics. Guidelines are extrapolated from recommendations for catheter-related thrombophlebitis and based on expert opinion [ ]. The use of anticoagulation similarly has limited data and is based on expert opinion, with recommendations for its use in great central veins and not peripheral veins.
The mycotic aneurysm was originally described by Osler as a mushroom-shaped vascular lesion seen as an embolic complication of infective endocarditis. In current day, the terminology can be confusing as it is sometimes used as an all-encompassing term for three distinct categories of infected aneurysms: (1) IE-related (classically mycotic) aneurysms, (2) secondary hematogenous seeding of preexisting sterile aneurysms, and (3) direct trauma and inoculation of vessels leading to an infected pseudoaneurysm. Mycotic aneurysms that are sequelae of IE are discussed elsewhere ( Chapter 9 ). In PWID, the direct arterial damage occurs from erroneous arterial venipuncture or contiguous spread from SSTI into the arterial vascular wall creating a pseudoaneurysm. They are epidemiologically relatively rare, with an estimated prevalence of 0.03% among PWID; however, they are surgical emergencies [ ]. The common femoral artery is the most frequently affected site, with the majority of patients having swelling, pain, and erythema [ ]. Resection with autologous vein implantation was the most common surgical strategy, though in general there is high associated morbidity. CT angiography has become the diagnostic modality of choice. S. aureus is the most common causative organism, and adjunctive medical treatment involves IV antibiotics for 4–6 weeks.
Patient-centered care
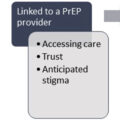
Providing acute medical care to persons with OUD is challenging but essential to reducing the associated morbidity and mortality. Persons with OUD infrequently access medical care due to stigma, criminalization of substance use disorder, and social determinants like poverty and rurality that are associated with OUD [ ]. Those who do seek care may be reluctant to disclose nonprescription and illicit opioid use for similar reasons leaving OUD undiagnosed despite frequent interactions with the healthcare system.
When hospitalized people who use drugs (PWUD) receive inappropriate treatment for cravings, withdrawal, and pain, patients may experience an exacerbation of substance use disorders leading to illicit drug use in the hospital and mistrust of providers and care teams [ ]. Without patient-centered treatment for OUD and related conditions, many fail to engage in their treatment plan, and elopement and discharges against medical conditions are a common challenge to inpatient treatment of OUD [ ]. A growing body of evidence suggests that a lack of patient-centered care contributes to adverse hospital outcomes such as untreated pain, illicit drug use, discharges AMA, and readmissions [ , ]. Furthermore, stigma, inadequate treatment of addiction (e.g., withdrawal), and attitudes and behaviors of nurses and physicians may create an unsafe environment for many with OUD [ , ]. To provide comprehensive, inpatient care for persons with substance use disorder, it is important to recognize and appropriately manage the following conditions.
Acute pain
Because OUD is associated with trauma, violence, and injection-related infections, persons with OUD are at a high risk for pain [ ]. Unfortunately, those with OUD are also at risk for inadequate treatment of acute pain. Providing analgesia in patients with OUD, especially in the context of MOUD, is challenging. Yet adequate treatment of acute pain is essential to patient care [ ]. Furthermore, pain is a risk factor for substance abuse and may trigger a relapse for those in recovery [ ]. In the context of the opioid epidemic, many clinicians have restricted opioid prescriptions for most of their patients, yet it is important for clinicians to appreciate that those with OUD are at greatest risk for untreated and/or inadequately treated acute pain.
Acute pain must be appropriately managed in order to support treatment of addiction and painful comorbidities such as bacterial infections. This will include continuation of MOUD and aggressive pain management incorporating both nonopioid therapies and nonpharmacologic interventions [ ]. Some medications to consider include nonsteroidal antiinflammatory agents and acetaminophen. Treatment of comorbid anxiety, depression, and withdrawal symptoms is imperative [ ]. When possible, an expert in pain management should be involved in the treatment plan. Anesthesia consultation can be useful for localized pain that can be alleviated by nerve block. Buprenorphine itself provides potent pain relief but its analgesic effects only last for 4–8 h—doses can be split three times a day for improved pain control.
Opioid-related medical conditions
Opioid-induced bowel syndrome
Chronic opioid use is associated with opioid-induced bowel dysfunction (OIBD), a spectrum of conditions including constipation, nausea, vomiting, delayed gastric emptying, and gastroesophageal reflux disease. Of the many manifestations of OIBD, pain is the most debilitating feature [ ]. Opioid-induced constipation (OIC) affects up to 57% of patients with chronic, noncancer pain using opioids [ ]. OIC is the result of opioids on the peripheral mu opioid receptors leading to impaired bowel motility, reduced stool frequency/straining, incomplete evacuation, and harder stool. A subset of patients may develop narcotic bowel syndrome (NBS), a paradoxical increase in abdominal pain which only partially responds to escalating opioids and is likely related to central hyperalgesia [ ]. Diagnosis of OIC and/or NBS both require exclusion of alternate diagnoses, which may be contributing.
First and foremost, treatment of OIBD requires empathy and communication to engage and educate patients on the link between their symptoms and opioids. Many pharmacologic options are available for OIC. Laxatives are often the first-line therapy and may improve stool frequency although there are no RCTs supporting their use. Through chloride channel activation, Lubiprostone improves nonmethadone OIC in noncancer pain patients and is superior to placebo in time to bowel movement [ ]. Prucalopride, a selective 5-HT4 agonist, has been shown to effectively treat OIC, but it is FDA-approved in the United States only for chronic idiopathic constipation, rather than OIC [ ].
Some opioid receptor antagonists have the potential to act peripherally and centrally, but their central effects have been attenuated to target OIC in the bowel. Oral naloxone acts predominantly on local mu opioid receptors in the gastrointestinal tract and has been shown to reduce laxative use with only mild withdrawal symptoms (e.g., yawning, shivering) [ ]. When used with oxycodone per rectum, Naloxone per rectum reduces colonic transit time [ ]. Naloxegol, a polyethylene glycol derivative (PEGylated) of naloxone, has limited ability to cross the blood-brain barrier and therefore targets the gastrointestinal tract to reduce transit time [ ]. Similarly, oral methylnaltrexone acts on peripheral mu opioid receptors to safely and effectively relieve OIC in those with chronic noncancer pain [ ].
Peripherally active mu opioid receptors antagonist (PAMORAs) alleviate OIC symptoms without antagonizing the central opioid receptors, which provide analgesia. Subcutaneous methylnaltrexone is a PAMORA approved for OIC in those receiving palliative care with an inadequate response to laxatives. Many novel therapeutics are under development and are expected to change the treatment paradigm for OIC in coming years.
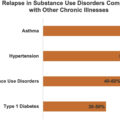
NBS is unique in its association with central opioid receptor manipulation, and, as a result, treatment with peripherally acting medications will be ineffective. For most, treatment will require reduction in opioid dosage and/or cessation. Although this is controversial, when used for patients with presumed NBS, opioid cessation has been shown to significantly improve abdominal and nonabdominal pain [ ]. According to the protocol used by Drossman et al., approximately 90% successfully stopped using opioids and 60% experienced improvement in symptoms. However, half who successfully ceased opioid usage resumed opioid use at 3 months, highlighting the challenges to treating opioid-related conditions [ ]. Several antidepressants and glutaminergic agents show promise in the treatment NBS but have not been thoroughly studied.
Hyperalgesia
Paradoxically, exposure to opioids can sensitize pain receptors leading to heightened pain responses, also known as opioid-induced hyperalgesia (OIH). By increasing pain perception in those on chronic opioid therapy, this phenomenon may lead to loss of opioid efficacy and/or the need for opioid dose escalation. OIH is not entirely understood but is thought to result from alterations in the peripheral and central nervous system (CNS) that sensitize pronociceptive pathways [ ]. While tolerance can be overcome by increasing doses of opioids, OIH cannot because it is a pain sensitization, which occurs in the CNS and peripheral nervous system [ ]. The resulting OIH pain may be the same as or distinct from the original pain. OIH is associated with a higher morphine dose and duration of therapy [ , ].
The diagnosis of OIH is challenging because the differential diagnosis includes opioid tolerance, exacerbation, or progression of an underlying disease process, and acute injury because the treatments are distinct [ ]. OIH is often diffuse and poorly defined in character and location, extending to areas beyond the primary pain process. OIH will not respond or will worsen with increasing opioid doses, whereas tolerance and an inadequately treated pain process will usually improve with dose escalation [ ].
OIH treatment should be managed by an expert in pain management and may include initial dose escalation to evaluate for tolerance. Subsequently, reducing or eliminating opioids, rotating opioids (buprenorphine, methadone), and adding opioid-sparing treatments may be considered [ ]. These adjunctive medications may include NMDA receptor antagonists and COX-2 inhibitors. Regardless of the approach, treatment of OIH is time and resource-intensive and will require frequent clinic visits, patient engagement, communication, and education in the outpatient setting.
Illicit and unprescribed opioid use in the hospital
Due to the nature of addiction, patients may continue to use illicit and unprescribed controlled substances while hospitalized. This may be related to withdrawal symptoms, emotional stress including anxiety, and/or pain. In one study, patients reported using drugs during hospitalization to manage pain and withdrawal symptoms and that these symptoms, when not managed medically, interfered with their treatment [ ].
Because illicit drug use is criminalized, many hospitalized patients may inject or swallow illicit drugs quickly and privately behind locked bathroom doors. This scenario is not conducive to harm reduction strategies and puts patients at risk of overdose and infection. IDU in the hospital also increases the risk of leaving AMA [ , ]. To prevent adverse outcomes associated with inpatient illicit drug use, providers must focus on treating the aforementioned symptoms related to abstinence (withdrawal, cravings) while managing comorbid mood disorders, anxiety, and pain [ ]. Although there are little data examining the impact of MOUD on inpatient illicit drug use, it is likely that timely initiation will reduce these high-risk behaviors.
Unplanned disposition and discharge against medical advice
Hospitalization has been described as a “reachable” moment for persons with substance use disorders because the inpatient setting allows access to social services, case management, and medical care for addiction and related complications [ ]. But persons with OUD and specifically PWID are more likely to disengage from medical care and leave the hospital prematurely [ , ]. As many as 30% of PWID leave the hospital AMA [ ]. Not only does this abbreviate inpatient services, such as antibiotics for injection-related infections, but also it precludes linkage to necessary outpatient services for substance use disorder and is associated with hospital readmissions and mortality [ , ]. One Canadian study found a threefold higher risk of death in the year following AMA discharge [ ]. There are, however, limited data on the frequency of these events in the United States in the context of the opioid epidemic and their impact on long-term morbidity and mortality. By focusing on the patients themselves and not the health system, we have missed an opportunity to restructure and align our healthcare models with the values and preferences of those with OUD, and specifically those who inject opioids. Even the term “against medical advice” is an adversarial term placing fault on patients rather than the health system that failed to engage them.
Ideally, patients at risk for AMA and other adverse hospital outcomes would be identified early in their hospitalization and receive frequent counseling, treatment of withdrawal and cravings by clinicians with expertise in Addiction Medicine, and MOUD, if indicated. In the absence of such services, the hospital serves as a “risk environment” for PWID [ ]. Preliminary data suggest that a patient-oriented treatment approach, including MOUD and adequate pain control, may reduce AMA, illicit drug usage, and other hospital risk behaviors allowing patients to engage in their care [ ].
Outpatient parenteral antibiotic treatment
Providing safe, effective outpatient parenteral antibiotic treatment (OPAT) for PWID is national priority due to rising rates of bacterial complications of injection drug usage. Because a peripherally inserted central catheter (PICC) provides durable venous access, PICC lines are critical to an effective OPAT plan. Guideline committees have previously discouraged the use of OPATs for PWID due to concerns of PICC misuse and diversion for illicit drug usage, and recent updates have accommodated case-by-case use [ , ]. But, in the context of the opioid epidemic and rising rates of injection drug usage, more providers and health systems are looking for alternatives to prolonged hospitalizations for completion of antibiotics. In a recent study conducted by the Infectious Diseases Society of America, a majority of the physician respondents (78%) reported caring for patients with infectious complications of injection drug usage [ ]. Respondents indicated a need for guidelines related to the safe, frequent provision of parenteral antibiotics for community-based treatment of infections.
Available data on OPAT protocols for PWID are limited in size and rigor [ , ]. Yet, according to a recent review by Suzuki et al., results are reassuring including reported OPAT completions rates of 72%–100% over the course of OPAT treatment, which may range from approximately 18 to 42 days [ ]. In some cases, OPAT participants with a history of IDU had a high mortality (up to 10%) and readmission rate (up to 41%). In at least two studies, PWID had favorable outcomes relative to other OPAT participants: one study found lower complication rates and another reported similar rates of readmissions, treatment failure, and death [ , ]. Because there are no RCT studies of OPAT in PWID, it remains unclear if these associations are due to the comorbidities of PWID, the OPAT protocol, or both.
Peripherally inserted central catheter
Several studies evaluating OPAT in PWID have documented adverse events related to PICC access. PICC complications occur in 3%–9% of patients [ , ]. Adverse events include deliberate misuse of a PICC line, which occurred in 2% in one population and as many as 11% left their medical respite facility with their intravenous access in place [ ]. Many unconventional methods have been used to deter and detect PICC tampering including the use of tape and/or dressing on valves and tubing of PICC catheters and daily PICC inspection. But it is worth noting that at least two studies have demonstrated that the rate of PICC-related infection in PWID is similar to those who do not [ ].
Models of care
As it currently stands, the majority of US healthcare systems are not adequately equipped to treat OUD and associated infections in an integrated manner [ ]. In some settings, especially in community and rural hospitals, specialty services such as addiction medicine or ID might be minimal. The most common situation is the lack of multidisciplinary integration between existing specialties and a lack of structural support to do so. Recently, there has been increasing recognition for the benefit of team-based specialty management for infections related to addiction, and unique models of care are now emerging.
For decades practitioners have understood the need for addiction medicine as a distinct medical field. After incremental and structural change throughout the latter half of the 20th century, it was relatively recently that the American Board of Addiction Medicine was created, in 2007, with the goal of establishing a board certified subspecialty. In 2015, the American Board of Medical Specialties (ABMS) approved this and the certification process is now open to any of the primary ABMS specialties. A strong relationship between ID and addiction medicine providers is essential for multidisciplinary treatment of patients with OUD and infections. Inpatient addiction consultation has been shown to reduce composite scores of SUD severity and number of days abstinence 1 month after discharge [ ]. Addiction treatment teams themselves can contain members of different backgrounds and skills. One model, termed the Improving Addiction Care Team (IMPACT) model, formed a team of addiction medicine providers together with social workers and peers with lived experience in recovery [ ]. The protocol included screening and diagnosis of SUD (including OUD), pharmacotherapy by physicians, CBT and mindfulness therapy by social workers, and group-based training with other healthcare workers such as nurses. The training groups included the creation of a Patient Safety Care Plan for patients with risk for illicit inpatient substance use or AMA risk, and another for PICC Community Safety Assessment to make a team-based informed decision on ability for discharge with an indwelling line. ID providers themselves can train and apply for buprenorphine waivers for inpatient and outpatient prescribing of MOUD. A survey of ID physician beliefs regarding OUD showed that a slim but significant majority believed that their field should be involved in MOUD prescribing [ ].
For those patients who do not require admission, the emergency room is an important point of contact for patients with OUD and infections. Similar to inpatient trends, OUD-related ER visits nearly doubled from 2005 to 2014 [ ]. Many patients who present with infections related to IV substance use have conditions such as minor abscesses and mild cellulitis that are routinely managed in the ER then discharged. A recent RCT evaluated the feasibility of buprenorphine initiation in the ER setting and found that retention in addiction care at 1 month was significantly higher for those either initiated on site or given medications for home induction [ ]. Follow-up data showed that the three groups appeared similar at the 2 month mark, though with methodological issues—all patients in the ED-initiated buprenorphine arm had to switch from primary care-based treatment to an OTP, perhaps affecting retention of care [ ]. Further study is required to assess generalizability and optimal implementation strategies.
Transitioning to the outpatient setting
As previously noted, there is no consensus on the appropriate timing of discharge for hospitalized persons given the aforementioned concerns about OPAT, and specifically PICC line use, for this population. In fact, when surveyed, as many as 41% ID physicians “frequently” manage the entire course of intravenous antibiotics for PWID in the inpatient setting, which may be 6 weeks or longer [ ]. And, following discharge, there are no best practices for patient care related to the delivery of antibiotics or the frequency of monitoring via a home health nurse or provider. For this reason, several health systems have implemented protocols aimed at reducing prolonged admissions for PWID at low risk for complication.
One academic hospital developed a risk assessment tool to determine if and when to transition patient care to OPAT. This study tool stratified PWID according to their risk of continued IDU [ ]. Patients deemed “low risk” were discharged to OPAT, while all others received the entire course of parenteral antibiotics in the hospital. By transitioning low-risk patients (27%) to OPAT, this hospital was able to reduce length of stay and direct hospital costs and create capacity for additional acutely ill PWID without increasing 30 day readmissions [ ]. Englander et al. developed the medically enhanced residential treatment (MERT), a hybrid residential treatment model integrating treatment for substance use disorder and parenteral antibiotics for patients requiring two or more weeks of antibiotics [ ]. Although this model was viewed as overall positive by key informants, few eligible patients enrolled in the MERT model of care. Among the barriers to MERT were concerns about restrictive policies in residential treatment and stigma from staff [ ]. This is consistent with findings by Fanucchi and colleagues that hospitalized persons with substance use disorders are unlikely to want residential treatment although many are interested in pharmacotherapy for addiction [ ].
Prevention
Although some areas of patient care related to OUD are uncertain, many opportunities for infection prevention are evidence based. In 2015, approximately 9% of new HIV infections were attributed, at least in part, to IDU [ ]. Fortunately, HIV preexposure prophylaxis (PrEP) is an effective HIV prevention option for adult PWID at substantial risk for HIV. HIV PrEP should be offered to persons who are injecting drugs regardless of recent treatment for addiction.
Because infectious diseases disproportionately affect PWUD, vaccination is an additional public health strategy to improve health outcomes for this population. Hepatitis B and C are acquired through sharing of needles and drug preparation equipment, but only Hepatitis B can be prevented through vaccination. Hepatitis B vaccination is, therefore, recommended for all current or recent injection drug users. Recently, there has been an increase in Hepatitis A outbreaks among PWID through both fecal-oral and percutaneous transmission. Because Hepatitis A vaccination is highly effective in preventing and abating such outbreaks, all PWID and all persons with homelessness should receive Hepatitis A vaccination [ ]. Due to poor hygiene and close living quarters, other vaccine preventable infections such as influenza can be devastating for PWID. For this reason, all PWID should receive evidence-based vaccination according to the Advisory Committee on Immunization Practices guidelines [ ].
Future directions
Research on optimizing care for OUD and its downstream infections is burgeoning. This is in part derived from need in the context of the ever-worsening opioid crisis in the United States. Novel treatment strategies could one day preclude the need, in certain infections, for intravenous therapy and its attendant challenges of requiring a PICC line for administration. Two major trials have been recently been published regarding use of oral antibiotics traditionally treated for long courses with intravenous antibiotics: one for a variety of bone and joint infections and another for partial oral treatment of infective endocarditis [ , ]. Both were pragmatic studies that demonstrated noninferiority of their primary endpoints using a variety of oral antibiotic regimens with and without rifampin use. However there were very few participants with IDU in either trial affecting their generalizability, and this will be crucial to be studied further. Long-acting injectable lipoglycopeptides have demonstrated noninferiority for SSTI treatment, at times with single dose treatment [ ]. Preliminary clinical data suggest its potential effectiveness in treatment of osteoarticular infections with randomized trial data needed [ ].
There is a profound gap between the patient need and the availability of prescribing providers for MOUD—in 2012, it was estimated that this gap was approximately one million people, and it has potentially widened in the context of the worsening opioid crisis [ ]. Providers of all specialties that have contact with people with OUD should apply for buprenorphine waivers for outpatient prescribing. Midlevel providers will play an important role in addressing the current need as well. All physicians with a primary ABMS specialty are permitted to sit for Addiction Medicine subspecialty boarding, for those who would like to pursue expertise in the field. Current study of the benefit of MOUD prescribing on inpatient ID consult services is promising and could serve to provide unified continuity for subacute infectious treatment and addiction treatment for patients with conditions such as infective endocarditis. Ultimately, full multidisciplinary integration of health systems in the inpatient setting for the treatment of addiction and infection will be the best modality for improving care.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree