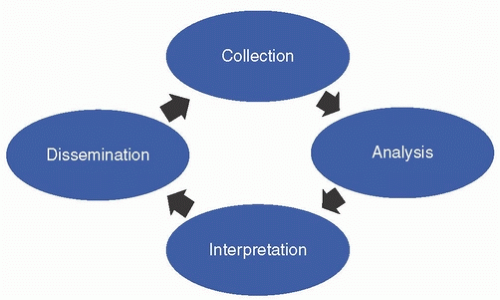
HISTORY OF U.S. INFECTIOUS DISEASE SURVEILLANCE
National surveillance for infectious diseases began in 1878 when the United States Congress passed the Port Quarantine Act authorizing the U.S. Marine Hospital Service to collect cases of cholera, smallpox, plague, and yellow fever from U.S. Consulates overseas for quarantine purposes to prevent introduction and spread in the United States. In 1893, the Interstate Quarantine Report expanded to weekly publication to include cases from states and municipalities. In 1902, the U.S. Congress reorganized the U.S. Marine Hospital Service to the U.S. Public Health Service (USPHS) and authorized the U.S. Surgeon General to provide standard forms for collection, compilation, and reporting at the national level. In 1912, State and Territorial Health Authorities and the USPHS agreed to formalize state to federal reporting of immediate telegraphic reporting of five infectious diseases and monthly reporting by letter of an additional 10 diseases. Subsequently, at the 1950 annual meeting of U.S. State and Territorial Health Officials, it was agreed that state and territorial epidemiologists would determine with the USPHS the list of national notifiable diseases at an annual meeting. This was the initiation of the U.S. Council of State and Territorial Epidemiologists. In 1961, the Centers for Disease Control and Prevention (CDC) assumed responsibility for the collection of these nationally notifiable disease data from physicians and laboratories and the publication in the CDC’s
Morbidity and Mortality Weekly Report. These data are currently used for measurement of incident endemic disease, outbreak recognition, assessment of prevention/control measures, allocation of public health resources, and contribution to epidemiological understanding of new and emerging pathogens.
1Surveillance of healthcare-associated infections (HAIs) has been identified as a key strategy in preventing infections in healthcare settings. In the 1970s, the Study on the Efficacy of Nosocomial Infection Control (SENIC) identified that the impact of hospitals developing intensive surveillance and prevention programs could prevent up to onethird of HAIs.
2 Following this report’s findings, the CDC also recommended surveillance as a key strategy for prevention of HAIs. The CDC’s National Healthcare Safety Network (NHSN) is the current national surveillance system for HAIs. It was formed in 2005 as a combination of the National Nosocomial Infections Surveillance System (NNIS), the National Surveillance System for Healthcare Workers, and the Dialysis Surveillance Network. The NNIS was initiated in 1970 in order to describe the epidemiology of HAIs and provide national level comparative rates for these outcomes. Submission of HAI surveillance data into the NNIS and eventually NHSN was voluntary until 2006-2007, when many states began requiring reporting of HAIs,
3 and later became publicly reportable as part of the U.S. Centers for Medicare and Medicaid Services (CMS) quality assurance programs. As part of this requirement, healthcare facility-specific surveillance data for certain HAIs must be reported to CMS; these include the following: central line-associated bloodstream infections (CLABSI, as of 2011 for intensive care unit [ICU] events, 2015 for events in medical and surgical wards), catheter-associated urinary tract infections (UTI) (CAUTI, 2012 for ICU events, 2015 for events in medical and surgical wards), surgical site infections (SSIs) after colon surgery and abdominal hysterectomy (2012),
Clostridioides difficile infections (CDI, 2013), and methicillin-resistant
Staphylococcus aureus (MRSA) bacteremia (2013).
4 This performance is now available on the Hospital Compare Web site.
5 The full cohort of surveillance components that are currently available within NHSN include biovigilance component (eg, transfusion-associated events), dialysis component, healthcare personnel (HCP) safety component (eg, HCP exposures and immunizations), long-term care facility component, outpatient procedure component, as well as the patient safety component, which has modules for device-associated infections (eg, CLABSI, CAUTI, ventilator-associated events [VAE]), procedure-associated module (eg, SSI), antimicrobial use and resistance module, and the multidrug-resistant organisms and
C difficile infection module.
6NHSN is considered the premier surveillance system in the United States for HAIs, defined broadly as a localized or systemic condition that results from adverse reaction to the presence of an infectious agent(s) or its toxin(s) and that was not present or incubating at the time of admission to the healthcare system.
7 The system offers a secure, Internet-based surveillance system for submission of both
patient and HCP safety surveillance systems. Standardized definitions are provided by the CDC, and data are collected and reported actively by infection preventionists. The CDC publishes the data as standardized infection ratios (SIRs) annually. NHSN provides infection rates or ratios with some risk adjustment primarily for device-associated infections and SSIs. Reporting into a national database allows acute care hospitals to benchmark their data to other hospitals, provides information on the scope of healthcareassociated pathogens, and provides data on the frequency and antimicrobial susceptibility of pathogens.