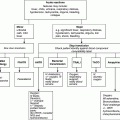
Chapter 20 Tim Collyns1 and Elankumaran Paramasivam2 1Leeds Teaching Hospitals Trust, St James’s University Hospital, Leeds, UK Patients with many haematological disorders have an increased susceptibility to infections. This may be due to disruption of the patient’s host defences by the underlying condition and/or the subsequent haematological treatment. Some examples are listed in Table 20.1; however, the spectrum of infectious diseases which may be involved varies with the type and severity of the haematological condition and the associated therapy [1–3]. It is also related to the infectious agents which are circulating in the patient’s surrounding environment and community and to which they have been exposed to. Table 20.1 Some specific infections associated with, and/or more severe in, specific conditions [1–3]. Depending on the haematological disease, patients may present with more than one infectious complication, either concurrently or consecutively. Patients may require critical care level support due to the systemic sequelae of an infection, or they may acquire certain infections while in the critical care environment. This chapter outlines some of the more common scenarios in the critical care setting and approaches to their diagnosis and successful management. Infectious complications contribute significantly to the overall morbidity and mortality of haematological diseases; hence, there will usually be local guidelines in place which should be consulted as required. This is the archetype of an infectious complication in the setting of haematological diseases. Standard, internationally applied definitions are available (Table 20.2), but there may be local variation in interpretation of both neutropenia and fever [1, 2, 4]. Diagnostic criteria for assessing sepsis severity are also outlined in Table 20.2 [5, 6]. The National Institute for Health and Clinical Excellence (NICE) in the UK has recently issued guidance for the prevention and management of neutropenic sepsis – in which the criteria for a diagnosis of sepsis includes a fever greater than 38°C alone, while neutropenia is defined as the patient’s neutrophil count being equal to, or less than, 0.5 × 109/L [4]. Table 20.2 Diagnostic criteria. Adapted from [1, 2, 4–6]. Neutropenic fever often arises in those with haematological malignancy undergoing chemotherapy. The absence of neutrophils, coupled with disruption of skin and mucosal barriers, predispose the patient to infection. The risk is inversely proportional to the absolute count, and 10–20% of patients with a neutrophil count less than 0.1 × 109/L will have a bloodstream infection. Fever is an early, albeit non-specific, sign of infection, although classic symptoms and signs may be reduced or absent [1, 2]. Only 20–30% of neutropenic fevers are due to clinically identified infection [2]. The aetiology of likely infecting organisms varies with length of neutropenia, previous or current antimicrobial therapy, as well as with clinical source. It is also influenced by the patient’s setting – whether in the community or in hospital and, if in the hospital, the particular unit’s microflora. Some attributes of the more common bacterial isolates are detailed in Table 20.3. Table 20.3 Some common bacterial pathogens [1–3, 6].
Infectious Complications in the Immunosuppressed Patient
2St James’s University Hospital, Leeds, UK
Introduction
Neutropenia
Viral infections
HSV reactivation
Bacterial infections (see also Table 20.3)
Gut translocation: Enterobacteriaceae (coliforms)
Line associated: staphylococci
Fungal infections
Candida species
Aspergillus species, most common Aspergillus fumigatus (other moulds)
Hypogammaglobulinaemia/impaired humoral immunity
Encapsulated bacteria: principally Streptococcus pneumoniae, also Haemophilus influenzae, Neisseria meningitidis
Sinopulmonary infections, +/− septicaemia
Lymphopenia/impaired cellular immunity
Herpesviruses, respiratory viruses
Listeria monocytogenes, Nocardia species
Mycobacteria: Mycobacterium tuberculosis and non-tuberculous
Cryptococcus species, P. jirovecii
Toxoplasma gondii reactivation
Asplenic/functionally hyposplenic
Encapsulated bacteria, principally S. pneumoniae, also H. influenzae, Capnocytophaga spp.; parasite infections, malaria, babesiosis
Acute leukaemias
If neutropenic, see preceding text. Patients with acute myeloid leukaemia (AML) or myelodysplastic syndrome (MDS) may be functionally neutropenic, i.e. detectable but ineffective neutrophils
Acute lymphocytic leukaemia (ALL): Pneumocystis
Chronic lymphocytic leukaemia (CLL)
Hypogammaglobulinaemic – see preceding text
CLL treatment (e.g. alemtuzumab, MabCampath®): wide range, including Pneumocystis, CMV reactivation
Multiple myeloma
Functionally hypogammaglobulinaemic – see preceding text
If neutropenic – see preceding text
Sickle cell disease
Functionally hyposplenic – see preceding text
Salmonella osteomyelitis
Iron overload (e.g. thalassaemias) and/or iron chelator therapy such as desferrioxamine
Yersinia spp.; other bacteria may have increased pathogenicity in presence of iron-rich milieu fungal infection (Zygomycetes)
Haemopoietic stem cell transplant (HSCT) recipients
Relative risks vary considerably with source/type of transplant and conditioning regime, as well as underlying disease and previous treatment. Allogeneic recipients are more likely to have infectious complications, and be at risk for longer, than autologous recipients. In the former, the presence of significant GVHD notably increases the probability of certain infectious complications
Conventionally for allogeneic recipients: 3 phases post HSCT
Pre-engraftment (day 0 usually to < day 30)
Neutropenic: see preceding text (also present post autologous HSCT though usually less prolonged)
Early post-engraftment (up to day 100)
Lymphopenic – see preceding text
Specific risks include CMV reactivation (seropositive recipient and/or donor)
Respiratory viruses, including adenovirus
BK virus (haemorrhagic cystitis)
Aspergillus species and other moulds
Pneumocystis
T. gondii (seropositive recipient)
Late post-engraftment (day 100 to reconstitution of immune function – usually around 18 months post allogeneic HSCT but delayed in presence of GVHD/associated treatment)
Sinopulmonary infections: S. pneumoniae, H. influenzae, Pneumocystis, Aspergillus species and other moulds
Varicella zoster virus (VZV) (seropositive recipient)
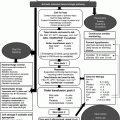
Neutropenic fever
Neutropenic fever
Absolute neutrophil count <0.5 × 109/L blood or <1 and expected to fall to <0.5 within the next 48 h
Single temperature ≥38.3°C (i.e. 101°F) or ≥38°C for 1 h or more
(NB NICE definitions: ANC < 0.5, temperature > 38°C [4])
Sepsis
Infection (suspected or proven) coupled with deranged parameters indicative of systemic response, including:
Pyrexia (>38.3°C) or hypothermia (<36°C)
Tachycardia (>90 beats/min)
Tachypnoea (>30 breaths/min)
Significant oedema or positive fluid balance (>20 mL/kg/24 h)
Abnormal blood tests:
Leukocytosis* (>12 × 109/L) or leukopenia* (<4)
Thrombocytopenia* (platelet count <100 × 109/L)
(*NB often not applicable in haematology patient setting)
Significantly elevated CRP (or PCT)
Hyperglycaemia (plasma glucose >7.7 mM/L) without diabetes
Arterial hypotension (see also severe sepsis)
Organ dysfunction or tissue perfusion markers (see also severe sepsis)
Severe sepsis
Sepsis with dysfunction or hypoperfusion of organs not primarily infected, e.g. lactic acidosis, oliguria (<30 mL/h or <0.5 mL/kg/h), altered mental state and/or hypotension (systolic pressure < 90 mmHg, mean arterial pressure <70 mmHg or drop of > 40 mmHg from baseline) – which is correctable with fluid resuscitation
Organ dysfunction can be recorded/monitored using validated scoring systems, such as Multiple Organ Dysfunction (MOD) or Sequential Organ Failure Assessment (SOFA) scores
Septic shock
Sepsis-induced persistent arterial hypotension which requires pressor therapy to correct
Refractory septic shock
Septic shock that lasts >1 h despite the use of pressor therapy
Gram-negative bacilli
Enterobacteriaceae (coliforms)
e.g. Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., other genera such as Citrobacter spp., Serratia spp.
Likely sources: gut translocation; urinary tract, respiratory tract (vascular catheter)
Treatment: usually susceptible in vitro to suitable broad-spectrum β-lactams such as piperacillin–tazobactam, carbapenems (imipenem/meropenem) and ceftazidime, although resistance is increasing. Carbapenems most reliable against antibiotic-resistant isolates, such as extended-spectrum β-lactamase (ESBL) producers or those with derepressed chromosomal AmpC gene – though carbapenemase-producing isolates are also emerging. Aminoglycosides usually also active and synergistic with a suitable β-lactam
Non-lactose-fermenting Gram-negative bacilli
e.g. Pseudomonas aeruginosa, other Pseudomonas spp., Acinetobacter spp., Stenotrophomonas maltophilia
Likely sources: vascular catheter, respiratory tract (gut translocation of P. aeruginosa)
Treatment: less predictable sensitivity patterns than Enterobacteriaceae. Suitable empirical febrile neutropenia regimes need effective anti-P. aeruginosa activity
S. maltophilia intrinsically resistant to the carbapenems. Antimicrobial of choice for this organism is usually co-trimoxazole (at mid-dose: e.g. 1.44g twice daily for 75kg patient)
Gram-positive bacilli
Staphylococci
e.g. Staphylococcus aureus, coagulase-negative staphylococci (including Staphylococcus epidermidis)
Likely sources: vascular catheter, skin and soft tissue infection (S. aureus), skin flora contaminant (coagulase-negative staphylococci)
Treatment: If meticillin susceptible, suitable β-lactam such as flucloxacillin. Carbapenems also active. If meticillin resistant, glycopeptide, linezolid, daptomycin
Streptococci
e.g. Streptococcus pneumoniae
Likely sources: respiratory tract, head (sinuses, ear, meninges)
Treatment: piperacillin–tazobactam, aforementioned carbapenems usually active (consider de-escalation to benzylpenicillin or amoxicillin), certain fluoroquinolones (e.g. levofloxacin or moxifloxacin, not ciprofloxacin), macrolide (e.g. clarithromycin), linezolid, glycopeptides
β-Haemolytic streptococci: Lancefield groups A, B, C, G
Likely sources: skin/soft tissue infection, gut translocation (Group B streptococcus)
Treatment: as for S. pneumoniae
Viridans streptococci ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access