Fig. 17.1
Phases of opportunistic infections among allogeneic HSCT recipients. EBV Epstein–Barr virus, HHV-6 human herpes virus 6, PTLD posttransplant lymphoproliferative disease . © Granted by Elsevier
a.
Viral infections: Herpes simplex virus (HSV), varicella zoster virus (VZV), community respiratory viruses, enteric viruses, human herpes virus-6 (HHV-6), etc.
b.
Bacterial infections: Gram-positive (Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus species, Enterococcus species) and gram-negative organisms (Klebsiella species, Pseudomonas aeruginosa, Escherichia coli), with resultant bacteremias as well as sinopulmonary, perirectal, gastrointestinal, and skin/soft tissue infections
c.
Fungal infections: Predominantly Candida and Aspergillus species
2.
One to four months post-transplant (early post-engraftment):
a.
Viral infections: Cytomegalovirus (CMV), HSV, VZV, community respiratory and enteric viruses, BK virus, and HHV-6, which can cause infection of the sinopulmonary, central nervous system, gastrointestinal, hepatic, and urogenital systems, depending on the causative organism
b.
Bacterial infections: Gram-positive and Gram-negative organisms, primarily arising from/involving the sinopulmonary system, the gastrointestinal tract, and skin/soft tissue
c.
Fungal infections: Candida, Aspergillus, and Cryptococcus species, Mucorales, reactivation of endemic fungi, typically involving the sinopulmonary, central nervous system, liver, spleen, mouth and/or skin/soft tissue; Pneumocystis jirovecii pneumonia (PCP) in patients on suboptimal PCP prophylaxis
d.
Protozoal infections: Toxoplasma gondii, which can affect the central nervous system or present in a disseminated fashion
3.
Four to twelve months post-transplant (late post-engraftment):
a.
Viral infections: VZV, community-acquired respiratory and enteric infections, and CMV infection in patients with GVHD and prior history of early posttransplant CMV reactivation/infection
b.
Bacterial infections: Encapsulated organisms (e.g., Streptococcus pneumoniae, Haemophilus influenzae, etc.)
c.
Fungal infections: Both yeasts and molds (e.g., Candida, Aspergillus, Cryptococcus species, Mucorales, etc.), particularly in those patients who remain on immunosuppressive therapy, have GVHD and/or CMV infection; Pneumocystis in patients on suboptimal prophylaxis
d.
Protozoal infections: T. gondii, which can affect the central nervous system or present in a disseminated fashion
4.
Greater than 12 months post-transplant:
a.
Viral infections: VZV, community-acquired respiratory and enteric infections, and CMV infection in patients with chronic GVHD and prior history of CMV reactivation/infection.
b.
Bacterial infections: Encapsulated organisms (e.g., S. pneumoniae, H. influenzae, etc.).
c.
Fungal infections: Both yeasts and molds, particularly in those patients who remain on immunosuppressive therapy have GVHD and/or CMV infection.
d.
Protozoal infections can occur late as well, again primarily in patients who remain on immunosuppressive therapy.
17.2 Empiric Antimicrobial Therapy and Evaluation of Neutropenic Fever
1.
For the first neutropenic fever (T ≥ 38 °C):
a.
Comprehensive fever workup to include the following, with additional testing as prompted by localizing signs/symptoms:
i.
Blood cultures from peripheral blood draw as well as all lumens of central catheter
ii.
Urine analysis (UA) dip/micro and urine culture
iii.
Sputum culture if patient is coughing and able to expectorate sample
iv.
Two-view chest X-ray (CXR) to evaluate for pulmonary infection
b.
Discontinue prophylactic antibiotic and begin empiric parenteral antibiotic therapy as soon as possible, and always within 1 h of the initial fever:
i.
Empiric antibiotic therapy should be sufficiently broad, providing coverage of P. aeruginosa, Enterobacteriaceae, and oral streptococci.
ii.
Options include cefepime (fourth generation cephalosporin), piperacillin/tazobactam, or an antipseudomonal carbapenem (e g., meropenem or imipenem).
iii.
Consideration of the local institutional antibiogram as well as any patient-specific history of prior drug-resistant bacteria is critically important in determining the empiric antibiotic selection.
iv.
For septic/clinically unstable patients, consider broadening empiric regimen to include an aminoglycoside (e.g., tobramycin 5 mg/kg intravenous (IV) once daily, adjusted for renal function; once-daily dosing is preferred) as well as extended Gram-positive coverage (see Sect. 17.2).
c.
For subsequent fevers:
i.
Frequent (at least daily), thorough clinical evaluation for signs or symptoms of new or emergent infection is imperative.
ii.
For T ≥ 38 °C, obtain blood cultures every 24 h for 2–3 days.
iii.
If fevers persist, blood cultures should be obtained in the context of clinical worsening and/or prior to any change to the empiric antibiotic regimen.
iv.
After initial defervescence with empiric antibiotics, recrudescent fever should be reevaluated with blood cultures and careful clinical assessment.
d.
Adjustment of empiric antibiotic regimen:
i.
If cultures are positive or if source of infection is defined, ensure regimen is appropriate based on pathogen susceptibility pattern and/or source.
ii.
Discontinue empiric antibiotic therapy once absolute neutrophil count (ANC) > 500 cells/mm3 if patient remains afebrile and provided there is no documented infection.
2.
Indications for use of empiric extended Gram-positive coverage for neutropenic fever:
a.
Add vancomycin for any patient with:
i.
Sepsis/unstable clinical condition, particularly for those patients with an established history of methicillin-resistant S. aureus (MRSA) colonization or infection, and not previously known to be colonized/infected with vancomycin-resistant Enterococcus (VRE)
ii.
Documented infection with a Gram-positive organism while awaiting results of identification and susceptibility testing (e.g., Gram-positive cocci in clusters or pairs/chains for patient not previously known to be VRE colonized/infected)
iii.
Skin/soft tissue infection
iv.
Suspected/established catheter-related infection
v.
Healthcare-associated pneumonia, while awaiting data from respiratory culture
b.
For patients known to be VRE colonized/infected, use daptomycin*as extended Gram-positive agent in the setting of sepsis and/or Gram-positive bacteremia (Gram-positive cocci in pairs and/or chains) while awaiting results of identification and susceptibility testing. Given the potential for myelosuppression with linezolid, daptomycin may be the preferred agent in this setting. *Note that daptomycin should not be used for the treatment of pneumonia, given its ineffectiveness in this setting; in the setting of possible/proven MRSA pneumonia, consider the use of vancomycin or linezolid.
c.
Blood as well as wound and sputum (when applicable) cultures should be obtained prior to adding vancomycin, daptomycin, or linezolid.
d.
Discontinue vancomycin, daptomycin, or linezolid after 72 h if no Gram-positive organisms have been cultured and patient has no evidence of shock, pneumonia, skin/soft tissue, or central venous catheter source, regardless of the presence or absence of fever.
3.
Management of persistent neutropenic fevers ( > 72 h after initiation of empiric antibacterial therapy):
a.
Frequent (at least daily), thorough clinical evaluation for signs or symptoms of new or emergent infection is imperative.
b.
Strong consideration for computed tomography (CT) chest to evaluate for opportunistic pulmonary infection.
c.
Consideration to broadening empiric antifungal coverage:
i.
For patients who are receiving fluconazole prophylaxis, change therapy to voriconazole (see Chap. 10 for dosing guidelines), or to an echinocandin (e.g., micafungin 100 mg IV q24 h; caspofungin 70 mg IV ´ 1, then 50 mg IV q24 h; or anidulafungin 200 mg IV ´ 1, then 100 mg IV q24 h) if azole-resistant candidiasis is suspected/documented.
ii.
If voriconazole is contraindicated (e.g., liver enzyme abnormalities, drug–drug interactions), alternatives include:
Lipid-based amphotericin product (3–5 mg/kg IV q24 h)
Echinocandin, though recognizing the inferiority of these agents for prophylaxis/treatment of mold infections
d.
For patients who are receiving posaconazole prophylaxis, obtain a CT chest, check serum galactomannan, and send a posaconazole level (if not yet sent). If CT chest is suspicious for fungal infection or if the serum galactomannan is positive, consider switch to alternative agent (e.g., voriconazole or lipid-based amphotericin product) and consult pulmonary service for consideration of diagnostic bronchoscopy and/or other diagnostic testing.
e.
If a patient is receiving voriconazole and there is clinical suspicion for invasive mold infection, entertain possibility of subtherapeutic voriconazole level or a voriconazole-resistant organism and consider empiric change to lipid-based amphotericin product (Ambisome® or Abelcet®). Voriconazole level should be checked prior to drug discontinuation (see Table 10.5).
4.
Clinical criteria necessitating removal of central venous catheters include:
a.
Septic patient with suspected line source
b.
Tunnel tract infection
c.
Failure of response (persistent bacteremia with positive blood cultures after 48 h of appropriate antibiotic therapy)
5.
Central venous catheters should be removed for positive blood cultures with the following organisms:
a.
S. aureus
b.
P. aeruginosa
c.
Candida species
d.
Multidrug resistant Gram-negative organism
e.
Mycobacterial species
17.3 Treatment of Common Specific Infections in the HSCT Population
Of paramount importance in the treatment of infections in the HSCT recipient is the ability to obtain an accurate diagnosis . Symptoms of infection may be nonspecific or even attenuated in the heavily immune suppressed HSCT recipient. Diagnosis of infection may require culture of blood or other body fluid, molecular diagnostic testing (e.g., polymerase chain reaction, PCR), radiographic study, invasive diagnostics to obtain tissue or other material, as well as careful ongoing assessment for change in clinical status.
1.
Herpes zoster (VZV) infection:
a.
Rate of occurrence is decreased with acyclovir (or a related congener) prophylaxis.
b.
Typically occurs 4–5 months post-transplant (or later in allogeneic recipients) and may be associated with visceral or central nervous system disease.
c.
May be localized to a single dermatome or disseminated (see Fig. 17.2). A thorough skin examination is recommended to evaluate for disseminated disease.
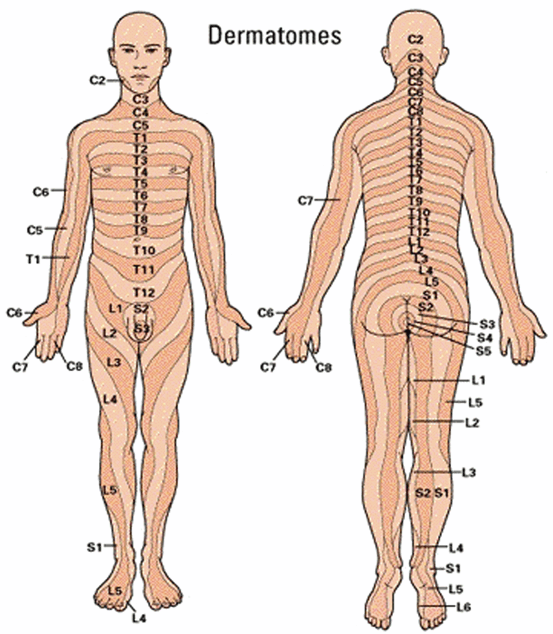

Fig. 17.2
Dermatome map for the determination of the extent of herpes zoster infection
d.
Oral antiviral therapy with acyclovir 800 mg orally (po) five times daily (adjust dose for renal function) is standard of care for lesions confined to a single dermatome. Valacyclovir (Valtrex®) achieves better therapeutic plasma levels against VZV and may be used as preferred alternative to oral acyclovir if cost does not preclude use (dosed at 1000 mg po three times daily (TID), renal dose adjustment as indicted; see Table 10.2).
e.
For severe herpes zoster infections (> 1 dermatome, trigeminal nerve involvement, visceral or disseminated disease), patients should be hospitalized and treated with intravenous acyclovir (10 mg/kg IV every 8 h, renal dose adjustment as indicated; see Table 10.2) until lesions have completely crusted and no new lesions are evident, then transitioned to an oral compound (acyclovir or valacyclovir) to complete the treatment course. Monitor for acute kidney injury and encephalopathy as possible adverse effects of high-dose, parenteral acyclovir.
f.
Acyclovir-resistant VZV is relatively unusual; if suspected, a viral culture should be obtained for phenotypic resistance testing, with consideration to use foscarnet (40 mg/kg IV every 8 h, renal dose adjustment as indicated) if resistance is proven or in the context of life-threatening infection while awaiting results of resistance testing, along with consultation to the infectious diseases service.
2.
HSV infection:
a.
Infection is largely related to reactivation in the post-transplant setting, and absent prophylaxis, occurs early (within the first month post-transplant) .
b.
Risk for infection is decreased with acyclovir (or a related congener) prophylaxis.
c.
HSV-1 infections most often present as severe mucositis and occasionally esophagitis, and less often with secondary infection of various organs in the context of viremia. HSV-2 infections are less common and typically affect the genital/perineal/buttocks region.
d.
For non-severe infection limited to the mucous membranes, oral antiviral therapy is usually adequate: acyclovir 400 mg po five times daily for approximately 7 days. If unable to tolerate oral medications, then use acyclovir 5 mg/kg IV every 8 h for approximately 7 days. Alternative therapy includes valacyclovir 500–1000 mg po two times daily (BID) for 5–10 days.
e.
In the case of suspected/proven visceral dissemination (e.g., encephalitis, hepatitis, pneumonitis), acyclovir 10 mg/kg IV every 8 h should be used as initial therapy, with duration typically 14–21 days, depending on clinical syndrome and clinical course.
f.
Select patients with frequently recurring outbreaks may require chronic antiviral suppression. Any of the following regimens is acceptable: acyclovir 400–800 mg po BID-TID or valacyclovir 500 mg po BID.
3.
HHV-6 infection:
a.
Infection is almost universally related to reactivation and occurs in 30–50 % of transplant recipients in the early post-HSCT period (2–4 weeks).
b.
Viremia is often asymptomatic, though has been purported to be associated with a variety of nonspecific presentations (e.g., bone marrow suppression, delirium) . A causal association with encephalitis is supported by numerous case reports and case series.
c.
When encephalitis is suspected, HHV-6 PCR testing (cerebrospinal fluid (CSF), blood) should be performed; magnetic resonance imaging (MRI) of the brain may reveal abnormalities, often involving the temporal lobes.
d.
Treatment is controversial, but for established encephalitis, foscarnet or ganciclovir should be used in therapeutic doses. Treatment decisions should be made on a case-by-case basis in consultation with the infectious diseases service.
4.
CMV infection:
a.
CMV infection can lead to end-organ disease in the HSCT recipient, manifesting as pneumonia, gastroenteritis, hepatitis , retinitis, encephalitis, etc .
b.
While detection of CMV by PCR in blood in the context of clinical signs/symptoms consistent with CMV disease is suggestive, more certain diagnosis typically requires diagnostic bronchoscopy and/or tissue biopsy. Furthermore, CMV PCR detection in blood is not fully sensitive for the detection of end-organ disease, particularly gastrointestinal disease . If CMV disease is suspected, tissue biopsy (for histopathology and viral culture) should be obtained when feasible.
c.
When CMV end-organ disease is suspected/proven, consultation with the infectious diseases service for patient-specific treatment recommendations is advised. First-line therapy for CMV disease is generally IV ganciclovir, with foscarnet reserved for cases with intolerance to ganciclovir (e.g., refractory cytopenias) or if ganciclovir resistance is suspected (e.g., if CMV viral load increases while on therapy for more than 2 weeks) or documented.
d.
Ganciclovir-resistant virus is an unusual occurrence in the HSCT population and most often occurs in patients who have had prolonged exposure to ganciclovir or valganciclovir.
e.
Treatment duration should be determined on a case-by-case basis, taking into consideration the severity of CMV disease and the immune status of the host. Typically, induction dosing should be given for at least 3 weeks until the CMV viral load is undetectable and symptoms of end-organ disease have resolved, with several weeks of maintenance IV ganciclovir or oral valganciclovir dosing thereafter (see Tables 10.3 and 10.4 for dosing).
f.
For CMV pneumonia, in addition to antiviral therapy, adjuvant immune globulin is generally recommended, largely based on small uncontrolled studies, though recent analyses have raised question about the value of this intervention:
i.
CMV-specific immune globulin has not been shown to be more effective than intravenous immunoglobulin (IVIG) and is more costly
ii.
The dose, frequency, and duration of IVIG for CMV pneumonia have not been well studied. Historically, IVIG dosing has been 500 mg/kg IV every other day for up to ten doses .
5.
Adenovirus and BK virus infections of the genitourinary (GU) tract:
a.
Both adenovirus and BK virus can result in hemorrhagic cystitis post-transplant.
b.
For patients who develop BK viral cystitis, the initial approach should consist of supportive care:
i.
Begin with antispasmotics (e.g., oxybutinin) or urinary tract analgesics (e.g., phenazopyridine).
ii.
Consider reducing immune suppression if feasible and begin continuous bladder irrigation if symptoms are not controlled with antispasmotics.
iii.
For patients who develop fulminant hemorrhagic cystitis, consider therapy with cidofovir; a variety of cidofovir dosing protocols have been reported in case reports and small case series (e.g., 1 mg/kg weekly to three times weekly without probenecid), with the goal of minimizing drug toxicity. Important adverse drug effects associated with cidofovir administration include nephrotoxicity as well as hematologic and ocular toxicity, and so careful monitoring is recommended in this setting.
iv.
Viral load quantification does not correlate with symptoms, and the clinical significance of the viral load is unknown.
c.
Adenovirus infection can manifest as hemorrhagic cystitis, but is significantly more likely than BK virus to result in disseminated and potentially life-threatening disease:
i.
Adenovirus can affect the lungs, gastrointestinal tract, liver, GU system and/or the central nervous system.
ii.
Patients who have a positive culture or PCR for adenovirus from their urine should have blood sent for quantitative adenovirus PCR.
iii.
For adenovirus viremic patients and/or in the setting of fulminant hemorrhagic cystitis, strong consideration should be given to systemic treatment, with cidofovir 5 mg/kg IV once weekly for 2 weeks and then every other week or 1 mg/kg three times weekly (renal dose adjustment as indicated). If systemic or disseminated disease (e.g., disease outside the GU tract) is suspected, add probenecid 2 g po 3 h prior to cidofovir dose, then 1 g po at 2 and 8 h after dose.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



