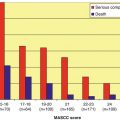
Fig. 1.1
Nature of febrile episodes in neutropenic patients
1.4 Microbiologically Documented Infections
Microbiologically documented infections also account for 25–30 % of febrile episodes in neutropenic patients (Fig. 1.1). The majority of these are monomicrobial (i.e., caused by a single pathogen), but polymicrobial infections are being documented with increasing frequency. Recent data show that ~15–25 % of bacteremias in neutropenic patients, including catheter-related infections, are polymicrobial [11–13]. Infections involving deep tissue sites are predominantly polymicrobial [14]. These include neutropenic enterocolitis, perirectal infections, complicated skin/skin structure infections, and pneumonia. The majority of polymicrobial infections are caused by multiple bacterial pathogens (gram-positive, gram-negative, and occasionally anaerobic organisms) although bacterial and fungal or bacterial and viral infections can also co-exist.
1.5 Sites of Infection
The most common and important sites of infection in patients with acute leukemia are listed in Table 1.1. Overall, the respiratory tract is the most common site of infection. Approximately 25 % of patients with acute leukemia will develop a pulmonary infiltrate during an episode of neutropenia lasting 10 days or longer. Other parts of the respiratory tract including the oropharynx, upper airways, and the paranasal sinuses are also frequent sites of infection. Most pulmonary infiltrates are secondary to an infectious process (bacterial and fungal organisms predominate) although it is often quite difficult to establish a specific microbiologic diagnosis. Noninfectious causes of pulmonary infiltrates such as alveolar hemorrhage and drug toxicity are much less common. Consequently, empiric therapy directed against anticipated pathogens is generally administered in such patients and can be modified if confirmatory microbiologic data become available. The management of patients with pulmonary infections/complications is discussed in detail else where. Approximately 15–20 % of patients with acute leukemia and neutropenia will develop a bloodstream infection. These include primary bacteremias and central line-associated infections. Gram-positive bacteria are isolated most often (~75–80 % of the time) with organisms colonizing the skin (e.g., Staphylococcus species, Bacillus species, Corynebacterium species) being predominant [13–16]. In patients with oral or intestinal mucositis, viridans group streptococci (VGS), enterococci (including VRE), and enteric gram-negative organisms are common pathogens [17, 18]. The frequency of gram-negative bacteremia is lower in patients receiving antibacterial prophylaxis with agents such as the fluoroquinolones, than in patients not receiving prophylaxis [19, 20]. Fungemias occur ~4–6 % of the time, are caused most often by Candida species, and are often associated with indwelling central venous catheters [21–24]. With the exception of Fusarium species, invasive mold infections seldom cause fungemia [25, 26]. A small proportion of bacteremic infections are caused by nontuberculous mycobacteria [27].
Table 1.1
Common sites of infection in patients with acute leukemia
Site of infectiona | Frequency (%) |
|---|---|
Respiratory tractb | 30–40 |
Bloodstreamc | 15–20 |
Urinary tract | 10–15 |
Skin and skin structure | 8–10 |
Intestinal tractd | 5–8 |
Other sitese | 10–15 |
Urinary tract infections are documented in 10–15 % of patients with acute leukemia, especially in patients requiring the placement of short-term or long-term urinary drainage catheters. Gram-negative bacterial pathogens such as Escherichia coli predominate although Candida species are not uncommon in patients with urinary catheters, stents, or other devices and in those that have received multiple courses of broad-spectrum antibacterial therapy for previous episode of neutropenic fever.
Common skin and skin structure infections include cellulitis, infections at phlebotomy or other puncture wounds, and surgical site infections in patients who have undergone recent surgery. Uncommon, but more serious infections include pyomyositis (occasionally caused by E. coli) and necrotizing fasciitis [28]. These conditions usually require surgical intervention in addition to antimicrobial therapy. Even less common are primary cutaneous mold infections [29, 30].
Infections along the gastrointestinal tract are not uncommon. Prior to the frequent use of antifungal prophylaxis, thrush and esophagitis caused mainly by Candida species (occasionally by herpes viruses) were commonplace. Azole and echinocandin prophylaxis has rendered these infections largely of historical interest. Neutropenic enterocolitis (typhlitis) occurs primarily in patients with acute leukemia who receive therapy with agents (e.g., cytosine arabinoside, in combination with idarubicin or another anthracycline) that cause high-grade intestinal mucositis although it is being described with increasing frequency in patients receiving other mucotoxic antineoplastic agents such as the taxanes and vinorelbine [31–33]. Perirectal infections occur more often in patients with preexisting local lesions such as fissures and hemorrhoids [34]. True abscess formation is uncommon in patients with severe and prolonged neutropenia, but surgical drainage is almost always beneficial [35, 36].
1.6 Spectrum of Bacterial Infection
Recent epidemiologic data document a predominance of gram-positive pathogens from microbiologically documented infections [13–16]. Unfortunately, these data focus on monomicrobial bacteremic infections, and do not provide details from most other sites of infection, or from polymicrobial infections. This gives an incomplete and skewed view about the microbiology of these infections since bacteremias are caused most often by gram-positive organisms that colonize the skin, whereas infections at most other sites (lung, intestinal tract, urinary tract) have a predominance of gram-negative pathogens [14]. Additionally ~80 % of polymicrobial infections have a gram-negative component, and ~33 % are caused by multiple gram-negative species [11]. When all sites of infection and polymicrobial infections are taken into consideration, a substantially different picture emerges, with gram-negative pathogens being almost as frequent as gram-positive pathogens [14]. Indeed, some institutions are now reporting a predominance of gram-negative pathogens [37]. Knowledge of local epidemiologic patterns is critical as empiric regimens need to be designed with this information in mind.
1.6.1 Gram-Positive Organisms
The microorganisms isolated most often from neutropenic patients are listed in Table 1.2. The most commonly isolated organisms isolated overall are the coagulase-negative staphylococci (CoNS) [38]. These organisms are generally of low virulence and seldom cause serious or life-threatening infections. Catheter-related bacteremias are the most common infections caused by CoNS. These can often be treated with antimicrobial agents without removal of the offending catheter, although some infections may recur if the catheter is retained [39]. The one exception is Staphylococcus lugdunensis, which resembles S. aureus in virulence, and infections caused by this species need to be managed like those caused by S. aureus [40–42]. Other gram-positive organisms that frequently colonize human skin and often cause infections in patients with leukemia include Bacillus species, Corynebacterium species, and Micrococcus species [43–49]. Like CoNS, the most common infection caused by these organisms is catheter-related bacteremia. Occasionally more serious infections such as pneumonia, endocarditis, endophthalmitis, and meningitis develop. The organisms are uniformly susceptible to vancomycin, linezolid, and daptomycin, whereas susceptibility to other agents is variable. Most patients respond to appropriate antimicrobial therapy, and infection-related mortality is low. It is not clear whether removal of the infected catheter is always necessary for response; however, recurrent infections seem to be more frequent if the catheter is retained [45, 48, 50]. As mentioned earlier, infections caused by S. aureus are more virulent and are associated with substantial morbidity and mortality [51]. In some cancer treatment centers, ~40–60 % of these organisms may be methicillin resistant, although institutional and regional differences do occur, with resistance rates <10 % in the Netherlands or Scandinavian countries. Some of these isolates have also developed tolerance or reduced susceptibility to vancomycin (the so-called MIC creep), and slow response to, or overt failure of vancomycin therapy has been reported especially in infections caused by organisms with vancomycin MICs of >1.0/ml [51–56]. In a recent study of MRSA bacteremia in cancer patients from a comprehensive cancer center, a high treatment failure rate for vancomycin (52 %) was demonstrated, and a vancomycin MIC of >2/ml was found to be an independent factor for vancomycin failure [57]. Based on this and similar reports, the current recommendation is to consider therapy with alternative agents such as linezolid or daptomycin for infections caused by organisms with reduced susceptibility to vancomycin [58–60].
Table 1.2
Microorganisms isolated most often from neutropenic patients
Gram positive |
Coagulase-negative staphylococci |
Staphylococcus aureus (including MRSA) |
Viridans group streptococci |
Enterococcus species (including VRE) |
Corynebacterium species |
Beta-hemolytic streptococci (groups A, B, G, and F) |
Stomatococcus mucilaginosus |
Gram negative |
Escherichia coli |
Klebsiella species |
Pseudomonas aeruginosa |
Enterobacter species |
Stenotrophomonas maltophilia |
Other Enterobacteriaceae |
Other non-fermentative gram-negative bacteria |
Fungal |
Candida species |
Aspergillus species |
Zygomycetes |
Fusarium species |
Viral |
Herpes simplex virus and varicella zoster virus (reactivation) |
Community respiratory viruses |
Alpha-hemolytic or viridans group streptococci are major components of human oral microflora. For many years, they were considered contaminants or organisms of low virulence, even in neutropenic patients. However, subsequent clinical experience has shown that they are responsible for serious, life-threatening infections in this patient population [61, 62]. The most consistent predisposing factor for infection by these organisms appears to be high-dose chemotherapy with agents such as cytosine arabinoside that induce severe mucosal damage, thereby facilitating entry of these organisms into the bloodstream [63]. Other probable predisposing factors include antimicrobial prophylaxis with fluoroquinolones that might encourage selection and overgrowth of these organisms and treatment of chemotherapy-induced gastritis with antacids or histamine type 2 (H2) antagonists [64–66]. Streptococcus mitis, S. sanguis, and S. salivarius are the predominant species [18, 67]. Bacteremia is the most common manifestation. In some patients, a rapidly progressive and disseminated infection (sometimes referred to as the streptococcal toxic shock syndrome) occurs involving the bloodstream, lungs, central nervous system, and skin [68]. Despite prompt and aggressive antimicrobial therapy, the mortality associated with this syndrome is 25–35 %. Of increasing concern are reports that 20–60 % of VGS are non-susceptible or overtly resistant to penicillin [18, 68]. This has limited the utility of penicillin G and other penicillins for the prevention and treatment of these infections. All isolates are currently susceptible to vancomycin, although tolerance has been described [18, 69–72]. The use of antibiotic combinations may be necessary, especially against tolerant organisms. These organisms are also susceptible to the newer-generation quinolones (e.g., moxifloxacin), daptomycin, and linezolid, but clinical experience with these agents is limited.
The enterococci reside primarily in the intestinal tract and cause a variety of infections such as bacteremia, urinary tract infection, endocarditis, intra-abdominal/pelvic infections, biliary tract infections, and occasionally pneumonia and meningitis. They are seldom primary pathogens but are seen most often following prolonged therapy with broad-spectrum cephalosporins or carbapenems to which they are intrinsically resistant. Increased use of vancomycin especially in neutropenic cancer patients was at least in part responsible for the emergence of vancomycin-resistant enterococci (VRE) globally, and these organisms now account for 15–30 % of all enterococcal isolates [4]. Fecal colonization with VRE is not uncommon in patients with acute leukemia and recipients of stem cell transplantation [73, 74]. Approximately 30 % of patients with VRE fecal colonization will go on to develop bacteremia or other significant infections with these organisms following chemotherapy, and some experts recommend the empiric use of agents with activity against VRE when such patients develop fever during an episode of neutropenia [10, 75]. Attempts at eradicating fecal colonization with VRE have been singularly unsuccessful. Consequently, infection control measures to reduce transmission of VRE are of overriding importance.
1.6.2 Gram-Negative Bacilli
The gastrointestinal tract serves as an important source of infection in neutropenic patients, with the predominant pathogens being enteric gram-negative bacilli. The use of antibacterial prophylaxis in high-risk patients including those with acute leukemia has led to a reduction in the frequency of documented gram-negative infections, although some centers are reporting a reversal of this trend [19, 20]. Nevertheless, gram-negative infections, when they do occur, are generally associated with greater morbidity and mortality than infections caused by their gram-positive counterparts. Multiple surveillance studies have shown that E. coli, Klebsiella spp., and P. aeruginosa remain the three most commonly isolated gram-negative organisms from neutropenic patients and collectively cause 65–75 % of microbiologically documented gram-negative infections [76–79]. Other Enterobacteriaceae such as Enterobacter spp., Citrobacter spp., Serratia spp., and Proteus spp. are less common, although institutional differences do exist. Despite the overall decline in the frequency of gram-negative infections in neutropenic patients, there has been an increase in the proportion of such infections caused by non-fermentative gram-negative bacilli (NFGNB) such as Acinetobacter spp., non-aeruginosa Pseudomonas spp., and Stenotrophomonas maltophilia [80]. Collectively, NFGNB now cause ~38 % of documented gram-negative infections, a proportion that has gradually increased over the years. The overall spectrum of infections caused by gram-negative bacilli is wide, with pneumonia, primary and catheter-related bacteremia, and urinary tract infection being common – Table 1.3.
Table 1.3
The spectrum of infections caused by Pseudomonas aeruginosa and other gram-negative bacilli
Bacteremia – primary and catheter related |
Pneumonia, empyema, lung abscessa |
Urinary tract infection – primary and catheter related |
Neutropenic enterocolitis (typhlitis) |
Perirectal infection/abscessa |
Skin and skin structure infection (ecthyma) |
Cholangitis/biliary tract infection |
Abdominal/pelvic/hepatic abscessa |
Otitis externa/mastoiditis |
Keratitis/endophthalmitis |
Osteomyelitis/septic arthritis |
Prostatitis |
P. aeruginosa is the most frequently isolated and the most important pathogenic NFGNB in this setting and causes between 15 and 20 % of all gram-negative infections [13–16]. Additionally, it is the most common gram-negative organism isolated from polymicrobial infections [11, 12]. These organisms have the propensity for developing resistance to antimicrobial agents by multiple mechanisms [81, 82]. A recent study demonstrated that the risk factors associated with multidrug-resistant P. aeruginosa infections were the use of a carbapenem as monotherapy for >7 days, a history of P. aeruginosa infection in the preceding year, and a history of chronic obstructive pulmonary disease [83]. Consequently, the antimicrobial stewardship program at our institution has targeted the prolonged use of carbapenem monotherapy, with a resultant decrease in the frequency of infections with MDR P. aeruginosa [84]. Stenotrophomonas maltophilia colonization/infection rates in neutropenic patients, especially those with acute leukemia and recipients of HSC transplantation, have increased considerably over the past two to three decades. Surveillance studies conducted at the University of Texas MD Anderson Cancer Center have documented an increase in the proportion of S. maltophilia from 2 % of all gram-negative bacilli isolated in 1986 to 7 % in 2012. Patients with prolonged neutropenia, those exposed to broad-spectrum antibiotics, especially the carbapenems, and those requiring mechanical ventilation have a higher risk of infection, although these infections are also seen in patients without traditional risk factors [85, 86]. The shift from trimethoprim/sulfamethoxazole (TMP/SMX) which has potent activity against S. maltophilia) to the fluoroquinolones (which are much less active against S. maltophilia) as the preferred agents for antimicrobial prophylaxis in high-risk neutropenic patients may also have contributed to the increase in infections caused by these organisms. TMP/SMX has been and remains the agent of choice for the treatment of infections caused by S. maltophilia, but in vitro resistance to it appears to be increasing [85, 87]. Ticarcillin/clavulanate also has reliable activity, whereas other beta-lactams such as ceftazidime, cefepime, and piperacillin/tazobactam have variable activity against these organisms. The newer quinolones such as moxifloxacin are more active than older agents such as ciprofloxacin and levofloxacin [80]. Minocycline and the novel glycylcycline – tigecycline, are also active against many S. maltophilia isolates [88]. Clinical experience with agents other than TMP/SMX and ticarcillin/clavulanate is limited. Combination regimens based on the susceptibility of individual isolates are often employed [89].
Other less common but important NFGNB include Acinetobacter spp., Achromobacter and Alcaligenes spp., Burkholderia spp., Chryseobacterium spp., and non-aeruginosa Pseudomonas species such as P. putida and P. fluorescens [90–96]. The clinical importance of these organisms has increased in recent years as they frequently cause outbreaks and MDR infections. Many outbreaks can be traced to sources such as contaminated dialysis fluid, chlorhexidine solution, deionized water, and mechanical ventilators.
1.7 Fungal Infections
Whereas bacterial infections predominate during the first 7–10 days of severe neutropenia, invasive fungal infections start to develop as neutropenia persists. Prior to the availability of agents like fluconazole, invasive candidiasis with or without hematogenous dissemination was common, with Candida albicans being the predominant species isolated. The frequency of invasive candidiasis has been substantially reduced with the routine usage of antifungal prophylaxis (azoles, echinocandins) in high-risk patients, with manifestations like Candida esophagitis, and chronic disseminated (hepatosplenic) candidiasis becoming increasingly of historical interest [97]. The use of some of these agents also led to the emergence of Candida spp. other than Candida albicans as frequent pathogens in this setting, although C. albicans continues to be the single most common species isolated [98, 99]. Regional differences have been documented with a preponderance of different Candida species at different centers [21, 22, 100–102]. These differences may represent divergent use of antifungal prophylaxis and/or geographic diversity. Consequently, local epidemiologic and susceptibility data should be used to guide empiric and targeted therapy. Other yeasts that are encountered in this setting include Trichosporon beigelii, Malassezia furfur, Saccharomyces cerevisiae, and occasionally Hansenula anomala [103, 104].
The risk of hematogenous dissemination with various yeasts is greater in patients who have indwelling vascular catheters and chemotherapy-induced mucositis or graft versus host disease [99, 105]. Currently, catheter removal in addition to appropriate antifungal therapy is recommended, although this strategy is by no means universally accepted [106–108]. Most C. albicans isolates maintain susceptibility to fluconazole and itraconazole. The newer triazole agents such as voriconazole also have potent activity against most pathogenic yeasts. The echinocandins appear to be effective for the treatment of candidiasis caused by most Candida species [106]. Treatment of candidiasis with polyenes is seldom necessary. Despite appropriate therapy, the overall mortality in cancer patients with candidemia (which is mainly due to the severity of the underlying disease) approaches 40 % [98]. Disseminated T. beigelii infections respond less frequently than disseminated candidiasis [104].
Invasive mold infections, due primarily to Aspergillus species, are the most frequent cause of serious, often life-threatening infections in patients with neutropenia that persists for more than 2 weeks [109]. Other risk factors include impaired cellular immunity, prolonged corticosteroid administration, allogeneic stem cell transplantation, and advanced age [103]. A. fumigatus is the predominant species isolated but non-fumigatus species of Aspergillus are emerging as significant pathogens [110–113]. The most common site of involvement is the lungs leading to invasive pulmonary aspergillosis (IPA). Other common sites of involvement include the paranasal sinuses, the central nervous system, the heart and pericardium, the liver, the kidneys, and occasionally, bones and joints. Cough, dyspnea, and hemoptysis are the classic manifestations of IPA but may be absent or muted in many patients due to severe immunosuppression leading to a blunted inflammatory response. Persistent fever in patients with prolonged neutropenia, despite appropriate antibiotic therapy, should raise the suspicion of invasive fungal infection including IPA. Most infections are diagnosed by computerized tomography (CT) imaging. Classic findings include nodular or wedge-shaped densities, the halo sign, and cavitary lesions [114, 115]. These findings change and evolve over time, and in response to therapy, consequently the performance of serial CT imaging has been found to be useful in monitoring patients with IPA. Aspergillus hyphae are angioinvasive in nature and result in release of fungal antigens into the bloodstream. Serologic testing to detect galactomannan or beta-D-glucan has been evaluated for the early diagnosis of invasive aspergillosis. The former appears to be more useful than the latter and may also be a predictor of outcome [116–120]. The use of these tests in conjunction with CT imaging has been discussed in various guidelines for the diagnosis and management of invasive fungal infections in neutropenic patients and HSCT recipients [121–123].
Several mold-active agents are now available for the prevention and treatment of invasive aspergillosis. These include amphotericin B and its lipid formulations, itraconazole, voriconazole, posaconazole, and the echinocandins. A detailed discussion of antifungal prophylaxis and therapy is beyond the scope of this chapter, but recent guidelines addressing these issues are available [121–125]. Although still uncommon, zygomycosis (mucormycosis) has emerged as an increasingly important infection in the past 15–20 years especially in patients with hematologic malignancies and HSCT recipients [99, 126, 127]. The increasing frequency of zygomycosis has at least in part been attributed to the use of voriconazole for various indications such as antifungal prophylaxis and empiric, pre-emptive or targeted therapy of invasive fungal infection [128–132]. The most common organisms isolated include Rhizopus species, Mucor species, and Cunninghamella species. Common sites of infection include the paranasal sinuses and orbit, the lungs, skin, and the central nervous system, with pulmonary manifestations being predominant in neutropenic cancer patients. Generalized dissemination occurs in up to 5 % of patients. Clinical features are often indistinguishable from other common mold infections. Early diagnosis of zygomycosis is important for timely therapeutic intervention, and ultimately, reduced mortality and improved survival. Conventional methods for laboratory assessment for zygomycosis include direct examination, cytopathologic examination, and histopathologic examination of respiratory and other relevant specimens. The use of immunohistochemical stains, fluorescent and in situ hybridization, or in situ polymerase chain reaction (PCR) may also be useful. Cultures from various specimens are often negative. There is increased reliance on diagnostic imaging such as CT of the paranasal sinuses and chest, which may reveal early findings even before the development of localizing symptoms [133, 134]. Unlike invasive aspergillosis where recent diagnostic and therapeutic advances have improved overall survival, the outcome of patients with hematologic malignancies who develop zygomycosis has not improved significantly [126, 127, 135]. Only two systemic antifungals have reliable activity against these organisms – amphotericin B and its lipid formulations and the new triazole, posaconazole. Recent guidelines advocate the lipid preparations of amphotericin B as first-line therapy, with posaconazole and combinations of caspofungin with lipid preparations of amphotericin B as second-line therapy [136, 137]. Surgery is recommended for rhinocerebral and soft tissue infections. Reversal of underlying risk factors is important. The duration of therapy remains unclear and should be guided by resolution of all associated symptoms and findings. Maintenance therapy and/or secondary prophylaxis should be considered in patients who remain severely immunosuppressed. Other uncommon but important molds that cause invasive infections in patients with hematologic malignancies include Fusarium species and Scedosporium species [138]. Unlike most other molds, fungemia is common in patients with fusariosis and may occur in ~40 % of patients [139]. Involvement of the paranasal sinuses, lungs, skin, and disseminated infection is also relatively common. Optimum therapy remains to be defined, and the overall outcome is poor. The incidence of Scedosporium infection appears to be increasing, with cases of S. prolificans generally occurring after 2000 [140]. As with fusariosis, optimum therapy remains to be defined, and the overall prognosis is poor.
1.7.1 Viral Infections
Viral infections per se are uncommon in patients with hematologic malignancies who do not receive HSCT. Most HSV and VZV infections in this setting result from reactivation of the latent viruses from previous exposure, and primary infections are rare [141, 142]. Most US adults are HSV-1 or HSV-2 seropositive, and reactivation can occur in up to 60 % of patients undergoing intensive chemotherapy for hematologic malignancies. Reactivation usually occurs soon after chemotherapy, while patients are still severely neutropenic, and much of the morbidity caused by oral mucositis has been attributed to HSV reactivation in this setting. Consequently, several guidelines recommend HSV prophylaxis in patients undergoing HSCT or remission induction therapy for leukemia [10, 143]. Reactivation of latent VZV also occurs but to a lesser extent, and the risk is considered insufficient to warrant routine prophylaxis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree