Class^
Median overall survival (months)
2-year survival (%)
1
58
76
2
37
68
3*
18
35
4*
11
15
5*
9
6
6*
4.5
4
Table 2
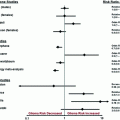
Hazard ration by age group in the EORTC/NCIC trial
Age, years (number of patients) | Hazard ratio | P value |
|---|---|---|
<50 (171) | 0.5 | 0.001 |
50–60 (220) | 0.63 | <0.05 |
61–65 (*) | 0.72 | 0.096 |
66–71 (*) | 0.8 | 0.34 |
Table 3
Treatment options for newly diagnosed elderly patients with glioblastoma
Treatment | Treatment parameters | Indication | Evidence |
|---|---|---|---|
Radiation therapy only | 40 Gy in 15 fractions | If MGMT methylation status not known or unmethylated | Evidenced-based |
Temozolomide monotherapy | 150–200 mg/m2/day x5 days every 4 weeks | If MGMT promoter methylation is present | Evidenced-based |
Best supportive care | Impaired performance status unable to care for oneself | Not evidenced-based | |
Combination therapy | Standard protocol of RT (60 Gy in 30 fractions) with concurrent TMZ followed by 6 cycles of postradiotherapy TMZ | Patients with good performance status (KPS > 60) | Not evidenced-based |
Clinical trial | Standard of care (RT + TMZ followed by TMZ) with an investigational agent | Patients with a good performance status (KPS > 60) and having undergone tumor resection (for tissue molecular correlates) | Investigational therapy |
Age is recognized as the most important prognostic factor for survival in GBM and survival declines after age 50 (a primary node point identified in the RTOG RPA classification system) [12]. Furthermore, there is a near linear decline in survival in patients with GBM greater than 50 years of age [3–5, 8]. Population-based studies of patients with newly diagnosed GBM show a mOS of 6 months in elderly patients, which is significantly lower than in younger patients [3, 5–7].
In addition to age, performance status (PS) is considered the second most relevant prognostic factor for survival in patients with GBM. Similar to patients >70 years of age, patients with markedly diminished or impoverished PS defined as an ECOG PS > 2 or a KPS < 60 have a mOS of 6 months or less. Because performance is so strongly correlated with survival, all current and most recent trials of newly diagnosed GBM only include patients with good performance status as defined by an ECOG performance score of 0–2 or a Karnofsky performance status of >60. These levels of performance imply independence in activities of daily living.
Two other relevant prognostic factors that are germane to elderly patients with GBM include tumor content of the DNA damage repair enzyme, methylguanine methyltransferase (MGMT), and the tumor mutational status of the isocitrate dehydrogenase 1 (IDH1) enzyme [13–17]. Patients with low tumor content of MGMT, a result of epigenetic silencing of the MGMT gene by promoter methylation, results in tumors with increased susceptibility to alkylator chemotherapy-induced injury. In elderly patients, the incidence of MGMT promoter methylated tumors is either higher (50 % as assessed by the German Glioma Network) or similar to that seen in younger adult patients (30–40 %) suggesting either no age dependence of MGMT methylation or possibly an increase with age [18–20]. Regardless MGMT promoter methylation status does not appear to adversely influence outcome in elderly patients with GBM. By contrast, IDH1 mutated gliomas currently defined as so-called secondary GBM, that is a GBM that arises from a lower grade glioma, have been demonstrated to have a more favorable outcome irrespective of treatment than the far more common (>90 %) primary GBM that arise de novo. The incidence of secondary GBM, however, decreases with age and, in contrast to MGMT promoter methylation, IDH1 mutations are age dependent and only rarely manifest in GBM of elderly patients (<2 %) [21]. The rarity of IDH1 mutated secondary GBM in the elderly may in part contribute to the above-mentioned poor overall survival.
Germane to treatment of elderly patients with GBM, geriatric oncologists recognize three categories of elderly patients based upon performance status, medical comorbidities, and age [22]. Frail elderly patients are defined by age >85 years (a category considered the oldest old), dependence in one or more activities of daily living, one or more medical comorbidities and one or more geriatric syndromes (defined as delirium, dementia, depression, osteoporosis, incontinence, falls, or failure to thrive). Physiologically, young elderly patients (as assessed by a geriatric scale) are defined by age <80 years, independence in activities of living, minimal to no medical comorbidities and no geriatric syndrome. The majority of clinical trials discussed below primarily relate to this category of elderly patient. The last category of elderly patients is those with a compromised PS that are dependent upon others in most or all activities of daily living. This category of elderly as well as younger patients with compromised PS is nearly always excluded from clinical trials due very limited survival.
2 Treatment
Several population-based studies document elderly patients with GBM receive less therapy than younger patients [3, 6, 7, 11, 23–25]. Of note the majority of published data on patterns of care in the elderly with GBM are derived before TMZ became available.
A SEER database analysis of 4,137 patients >65 years of age who were treated between 1994 and 2002 demonstrated that advancing age was associated with decreased use of resection, RT and chemotherapy, and with a diminished survival (mOS 4 months) [10]. A second SEER database analysis on 2,836 patients over the age of 70 showed that 86 % of patients received some form of treatment, but that only 46 % of patients underwent both surgery and RT [11]. In addition, another study reported that the rate of treatment with supportive care only increased with age [6]. A reason posited for diminished care in the elderly was the concern for increased toxicity from treatment with increasing age, patient preference, and the treating physician’s perceived treatment nihilism.
Until recently, there was a paucity of randomized clinical trials for the elderly GBM patient population and consequently the most appropriate treatment for this large cohort of patients with newly diagnosed GBM was ill-defined and controversial (Table 4). Two previous randomized studies in elderly GBM patients demonstrated that involved field fractionated radiotherapy (RT50: 50 Gy in 28 fractions) is superior to supportive care only (median survival 7 vs. 4 months) and that conventional fractioned RT (sdRT; total dose 60 Gy in 30 fractions) is comparable to hypofractionated RT (hypoRT; 40 Gy in 15 fractions) [6, 7]. These trials provided evidence to commend in elderly patients with GBM and deemed candidates for treatment that hypoRT should serve as the standard of care for this subpopulation. Several subsequent retrospective studies suggested an alternative treatment that is standard dose TMZ (sdTMZ) with deferred RT, however, these studies constituted low level of evidence [26, 27].
Table 4
Clinical trials in elderly glioblastoma
Trial (Reference) | Age (years) | Number | Treatment | Median overall survival (months) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
RT60 | RT50 | RT40 | RT34 | RT + TMZ | TMZ | BSC | ||||
EORTC/NCIC [1] | 60–70 | 173 | x | x | 10.9/11.8 | |||||
NCIC [7] | >70 | 95 | x | x | 6.1/5.6 | |||||
French [6] | >70 | 81 | x | x | 6.6/3.5 | |||||
NOA-08 [18] | >65 | 412 | x | x | 9.6/8.6 | |||||
Nordic [29] | 60–69 | 100 | x | x | x | 7.5/7.0/7.9 | ||||
>70 | 191 | x | x | x | 5.2/7.1/9.0 | |||||
ANOCEF [30] | >70 + Low PS | x | 6.0 | |||||||
ANOCEF [31] | >70 + Low PS | x + Bev | 6.0 | |||||||
A recent prospective randomized German study (NOA-08 study) compared up-front TMZ in a dose-dense regimen (ddTMZ is given at 100 mg/m2/day for 7 consecutive days every 14 days) versus conventional fractioned RT (RT60: 60 Gy in 30 fractions) to elderly patients with high-grade glioma [HGG] (defined as age >65 years, KPS ≥ 60, and tumor histology GBM or anaplastic astrocytoma) {median survival 8.6 months vs. 9.6 months} [18]. The primary endpoint was overall survival and the trial design was that of a noninferiority endpoint. Median overall survival in the ddTMZ arm was 8.6 months versus 9.6 months in the sdRT arm demonstrating noninferiority between these two treatment regimens. As a consequence of this study, an evidence-based conclusion would be that TMZ may be administered as an alternative to elderly patients with GBM as opposed to sdRT. What remains unclear notwithstanding the above-mentioned three randomized trials is how to treat elderly patients with GBM that have an impoverished performance, a not uncommon situation that accounted in part for the reduced number of patients enrolled in the NOA-08 trial. Of 584 patients screened for NOA-08, only 373 patients were ultimately treated per protocol, the 209 patients [36 %] deemed ineligible were primarily due to poor PS. In addition, whether the use of ddTMZ as used in the NOA-08 trial is superior compared to the standard 5-day TMZ regimen (sdTMZ) is unclear. The recently completed Radiation Therapy Oncology Group study, RTOG 0525 in patients with newly diagnosed GBM demonstrated no survival benefit to post-RT ddTMZ [19]. Further, the recently completed Medical Research Council trial of chemotherapy for chemotherapy naïve HGG in first relapse after treatment with surgery and RT showed no benefit to ddTMZ compared to sdTMZ [28]. Dose dense TMZ as acknowledged by the NOA-08 authors is more toxic and costly and likely no more efficacious compared to sdTMZ.
The very recently published Nordic randomized trial (342 patients enrolled, 291 randomized) that compared sdTMZ to sdRT to hypoRT (30 Gy in 10 fractions) in elderly GBM patients (defined as age >60 years and KPS ≥ 50) suggests sdTMZ is equivalent with respect to survival when compared to the hypoRT and superior to sdRT (60 Gy in 30 fractions) treatment arm [median survival 8.3 vs. 7.5 vs. 6 months] [29]. Based upon this prospective study, it would appear treatment with either sdTMZ or hypoRT is equivalent for elderly GBM patients and importantly evidenced-based.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree