Fig. 1
Figure shows a timeline of the various periods (over the last 3000–4000 years) of Black seed use that has led to the discovery of Thymoquinone. The first description of Black seed can be dated back to the Egyptian civilization where Nigella seeds were widely cultivated and used. The seeds also find mention in the Old Testament and later on in the Hadith of the Prophet of Islam (PBUH). The first scientific studies demonstrating the health benefits of Black seed and its oil were published in the 1960s. Isolation of Thymoquinone was documented in the 1970s that was followed by the first publications on its anticancer properties in the late 1990s. In recent times, research has focused on the development of novel analogues of Thymoquinone with better efficacy and superior bioavailability parameters
3 Black Seeds in Modern Times (Isolation and Characterization of Active Ingredients)
Black seed research has picked up pace in the last quarter of twentieth century and it is safe to say that most of the modern research has unfolded in the last 25 years or so. Table 1 shows the list of papers that are available on PubMed in relation to Black Cumin, Black seeds, Nigella Sativa, Thymoquinone, Black Seed Cancer, and Thymoquinone Cancer.
Table 1
Current status of literature on black seed and related research topics: a PubMed search
PubMed search key | Words returned search hits |
|---|---|
Black cumin | 725 |
Black seed(s) | 1100 |
Black seed cancer | 95 |
Black seed oil | 169 |
Black seed oil cancer | 29 |
Thymoquinone | 480 |
Thymoquinone cancer | 166 |
Thymoquinone analogue(s) | 29 |
The first reported study showing some impact of dietary Black seed consumption on Esophageal cancer came from an Iranian group (Ghadirian 1987). In this report, the authors evaluated dietary habits (including the consumption of black seed in diet) of a total of 1501 individuals, in 197 households, from 35 Iranian villages in different regions and its causal link to Esophageal cancer. While these studies did not give a very clear picture of the roles played by Black seed in cancer development or prevention, they certainly paved the way for future studies on Black seeds. Later on, Corder and colleagues showed that apoptosis by Lipopolysaccharide (LPS) or cortisol can be prevented by Black seed extracts (Corder et al. 2003). In another study, Hansen and colleagues showed that metabolic deregulation caused by LPS can be reversed using black seed extracts (Hansen et al. 2003). Having established the protective and immune stimulatory roles of Black seeds in various models, the research community moved toward exploring their anticancer effects. Salim and Fukushima were among the first group to demonstrate the chemopreventive benefits of a volatile black seed oil against chemically induced colon carcinogenesis in rat models (Salim and Fukushima 2003). These investigations drove further analyses into the constituents responsible for the anticancer activity of Black seed oil and ultimately resulted in the discovery of Thymoquinone.
4 Purification and Evaluation of the Anticancer Activity of Thymoquinone
Thymoquinone is the major bioactive component (~25 %) in Nigella sativa volatile oil. Over the years, Nigella sativa oil and its bioactive compound, Thymoquinone, have been shown to possess multiple health-beneficial activities, which include antitumor, anti-inflammatory, antibacterial, antidiabetic, antihypertensive, hyperglycemic, anti-oxidative, and immuno-modulation activities (Schneider-Stock et al. 2014).
Due to its multiple health benefits, extraction of Thymoquinone from Nigella sativa seeds was considered of prime importance and thus has received continuous attention from researchers and nutraceutical industry worldwide. There have been a number of methods developed for the extraction of Thymoquinone from Black seed oil including solvent extractions and hydrodistillation (Ghosheh et al. 1999). Some of these methods are time-consuming and costly and are not very environment friendly. Further, most of the solvent extraction methods impose a threat to consumers’ health if the organic solvents are not completely removed from the extractives. Improving on these methodologies, supercritical carbon dioxide fluid extraction (SFE) procedure has been patented recently that provides a better alternative for Nigella sativa seeds extraction US20120046366 A1. Advantageously, SFE offers the usage of nontoxic, nonexplosive, environmental friendly, cost-effective, time-saving, and selectivity-adjustable solvent (supercritical carbon dioxide fluid) in the extraction process. Furthermore, it also enables the oil extraction to be carried out under low temperature and oxygen-free condition. This feature is very crucial in the extraction of bioactive compounds that are highly susceptible to oxidative degradation, for instance, Thymoquinone. On the other hand, simultaneous fractionation by using SFE enables the concentration of targeted bioactive compound such as Thymoquinone to be conducted in a solvent-free as well as time- and cost-saving manner.
Having identified Thymoquinone as the major active component in Black seeds, researchers shifted their focus toward understanding their anticancer and disease preventing benefits. Thymoquinone is recognized to impact inflammation (Woo et al. 2012) and other diseases for which there are numerous outstanding reviews (Butt and Sultan 2010). Here we only focus on the anticancer activities of Black seed-derived Thymoquinone. Early indications for their anticancer benefits were chance findings. For example, Badary and colleagues while evaluating the preventive activity of Thymoquinone against nephrotoxicity induced by cisplatin observed an enhancement in the antitumor activity of the chemotherapeutic agents in rodents (Badary et al. 1997). Similarly, Worthen and colleagues showed anticancer activity of Thymoquinone and di-Thymoquinone (DIM) in cellular models of cancer (Worthen et al. 1998). Later on al-shabanah and colleagues showed that Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity (al-Shabanah et al. 1998). In other studies, Badary et al. have also showed inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice (Badary et al. 1999), suppression of 20-methylcholanthrene-induced fibrosarcoma tumorigenesis (Badary and Gamal El-Din 2001), and anticlastogenic activity against benzo(a)pyrene-induced carcinogenesis (Badary et al. 2007). Adding on to these findings, in their study Ghali-Muhtasib and colleagues demonstrated that Thymoquinone could trigger apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism (Gali-Muhtasib et al. 2004). Since these initial pathbreaking studies, a number of groups independently evaluated the anticancer activity of Thymoquinone as single agent or in combination with various chemotherapeutic agents against different tumor models. Table 2 below lists the different studies on Black seeds, Black seed oil, and Thymoquinone (either alone or in combination with different standard chemotherapeutics) that have been evaluated preclinically in different cancers.
Table 2
List of studies on black seed, black seed oil and thymoquinone in relation to cancer
Agent | Tumor models | Reference |
|---|---|---|
Black seed | Esophageal cancer | Ghadirian (1987) |
Colon cancer cells | Kamei et al. (1997) | |
Laryngeal cancer cells | Corder et al. (2003) | |
Black seed oil | Colon cancer | Salim and Fukushima (2003) |
Multi-organ carcinogenesis models | Salim (2010) | |
Hepatic metastasis models | Sorenson et al. (2011) | |
Thymoquinone | Breast cancer | |
Cervical cancer | ||
Cholangiocarcinoma | Xu et al. (2014) | |
Colon cancer | ||
Gastric cancer | Lei et al. (2012) | |
Hepatocellular cancer | ||
Intestinal cancer | ||
Leukemias (ALL, AML, CML, and others) | El-Mahdy et al. (2005) | |
Lymphomas | ||
Ovarian cancer | ||
Pancreatic cancer | ||
Prostate cancer |
5 Progress in the Development of Thymoquinone Analogues as Anticancer Agents
Bioavailability of any drug to the target cells, whether in vitro or in vivo, is critical for its optimal efficacy. If an agent does not possess optimal half-life and cannot reach target site (i.e., tumors), it is bound to have limited effects. Similar to many other natural dietary chemopreventive agents, Thymoquinone also possesses poor pharmacokinetic parameters. This is specially highlighted by the fact that the doses required for Thymoquinone to show in vitro cell death are in high micromolar range (usually >25 μM range for most cancer cell types). Therefore, despite the promising behavior shown in preclinical in vitro and in vivo cancer models, there are a number of bioavailability-related caveats that hinder the clinical translation of Thymoquinone.
There have been two distinct approaches to enhance the bioavailability of Thymoquinone: (a) nano-encapsulation and (b) development of Thymoquinone analogues with better efficacy and half-life. Effenberger and colleagues recently demonstrated that conjugates of thymoquinone with various monoterpenes, sesquiterpenes, and the cytotoxic triterpene betulinic acid possess anticancer activity (Effenberger et al. 2010). In these studies, the attachment of ester residues of various terpene alcohols to C(6) of Thymoquinone via spacers of variable length enhanced the growth inhibitory potential in multiple tumor cell lines (e.g., HL-60 leukemia, 518A2 melanoma, MDR KB-V1/Vbl cervix carcinoma, and MCF-7/Topo breast adenocarcinoma and nonmalignant foreskin fibroblasts). Similarly, Nair and colleagues described the potential of using nanobased formulations to package Thymoquinone to enhance its biological activity (Nair et al. 2010). Working further in this direction, Ravindran et al. developed a formulation in which Thymoquinone was encapsulated into biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticulates and the stabilizer polyethylene glycol (termed Thymoquinone-nanoparticle or simply TQ-NP). The resultant formulation demonstrated enhanced antiproliferative, anti-inflammatory, and chemosensitization potential against different cellular cancer models (Ravindran et al. 2010). These nanobased Thymoquinone showed superior cell kill as well as demonstrated a marked increase in their potential as a sensitizing agent to paclitaxel-induced apoptosis. In another study, a focused approach toward COX-2 docking was taken to develop analogues of Thymoquinone (Yusufi et al. 2013). The identified Thymoquinone analogues appended with gallate and fluorogallate pharmacophores showed superior activity against pancreatic cancer cell lines and also synergized with standard chemotherapeutic gemcitabine.
Our laboratory took a distinct approach and developed Thymoquinone analogues with the hope to enhance its potency and bioavailability (Banerjee et al. 2010). Using single-pot synthesis, we developed and tested several Thymoquinone analogues for their anticancer activity. Out of 27 analogues synthesized, 3 compounds exhibited superior activity than Thymoquinone. These compounds were further evaluated toward improvement of sensitivity to oxaliplatin and gemcitabine in pancreatic cancer cells and again were found superior to parent Thymoquinone in causing reduced cell viability and inducing apoptosis. Most importantly, the analogues showed greater retention in target organ (here pancreas) as detected using HPLC.
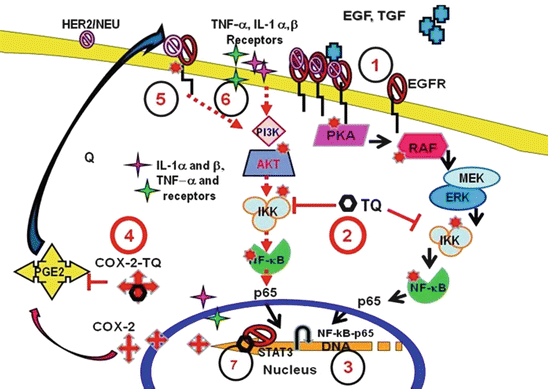
Despite the efforts on elucidating mechanism of action of Thymoquinone or its analogues, no clear single mechanism of action is yet to emerge. With each passing year, newer molecular targets of Black seeds, Thymoquinone, or their analogues are being defined that include apoptosis regulation, suppression of pro-survival transcription factors, blocking inflammatory response, and immune stimulation [the reader is referred to the excellent reviews on this topic (Schneider-Stock et al. 2014)]. Being natural products, they are bound to have a promiscuous mechanism of action. Our laboratory has primarily focused on NF-kB and COX-2 inhibitory properties of Thymoquinone and its analogues. Figure 2 is a summary diagram of the different mechanism that is thought to be supporting the underlying anticancer actions of Thymoquinone or its analogues. It is recognized that more work needs to be done in order to validate these targets in a broad spectrum of tumor models.