Tumor location
Method
Material
Central tumor with bronchial contact
Bronchoscopy
Conventional bronchial biopsy
Bronchial cryobiopsy
Brush cytology
Bronchoalveolar lavage
Peripheral tumor
Bronchoscopy
Transbronchial biopsy
Transbronchial cryobiopsy
Transbronchial needle aspiration
Brush cytology
Bronchoalveolar lavage
CT-guided percutaneous puncture
Transthoracic needle biopsy
Transthoracic needle aspiration
Peribronchial/mediastinal tumor
Bronchoscopy
Endobronchial ultrasound with TBNA
Classic TBNA
Bronchial as well as transbronchial biopsies have a high diagnostic yield as far as a sufficiently large biopsy forceps is used (open forceps diameter ~2 mm) and at least four tissue fragments are being taken. If the forceps is too small this will lead to artifacts, which hamper the morphological assessment and diagnosis. With a larger forceps, naturally, complication rates increase [2, 3], however, the respective complications, if they occur, are usually manageable if the appropriate clinical experience and equipment is at hand. Especially, the novel method of taking cryobiopsies, in which the tissue is frozen by a cryodevice at the tip of the endoscope in vivo and subsequently chunked out, is very effective and a reasonably secure method for the retrieval of large tissue fragments by means of endoscopy [4]. However, when applied in the transbronchial setting, this method is not very widely distributed and only available in specialized centers.
Percutaneous transthoracic needle biopsies, usually guided by computed tomography, complement the endobronchial methods for the diagnostic workup of peripheral lung lesions. The success rate of this method has been reported to range between 80–95 % [5, 6]. The negative predictive value of this diagnostic procedure is given with 84–96 %, false negative results can be expected in 2–4 % of the cases [7]. For this method, however, complication rates are somewhat higher than for endobronchial biopsy approaches and reach approximately 20–50 % [8–10]. However, complications (in most cases the occurrence of a pneumothorax) are usually manageable.
Apart from these methods, which usually guarantee that enough tissue material is at hand even when complex molecular analysis are required, techniques for obtaining cytology specimens also have their established place in the diagnostic workup in patients with lung neoplasms. Although cytology specimens are somewhat restricted with respect to tumor cell content and cellularity, optimized cytology workup procedures (see below) nowadays also allow for a broad array of diagnostic procedures (including morphology, immunohistochemistry, and molecular methods).
For those lesions which could not be reached by a direct biopsy approach (e.g., intrapulmonary tumors without bronchial contact, peribronchial nodal metastases) transbronchial needle aspiration techniques (TBNA) can be used either with or without ultrasound guidance [11, 12]. Larger needle diameters (e.g., 19G, inner diameter 0.69 mm) produce better results since more cellular cytology specimens and even small tissue fragments might be obtained. Endobronchial ultrasound (EBUS) guidance further improves the diagnostic yield, specifically when hilar and upper mediastinal lymph nodes are targeted (stations 2L, 2R, 10–12) [13]. The combination of EBUS with endosonographically-guided transesophageal fine-needle aspiration (EUS-FNA) increases the diagnostic accuracy even stronger in tumor patients, with both methods combined reaching a sensitivity of up to 96 % and a specificity of up to 100 % [14].
Bronchial brush cytology specimens can be taken prior or after the retrieval of bronchial biopsy specimens, the admixture of blood in the latter scenario does not compromise the quality of the probe [15, 16]. This method is specifically useful for tumors visually detectable by bronchoscopy. Bronchial lavage fluid can also be obtained prior or after biopsy retrieval, the use of 10–20 ml isotonic saline fluid has been recommended [15–17]. The obtained material is processed to produce smears and/or a cell block (see below), however, the diagnostic yield is worse than for brush cytology specimens. Bronchoalveolar lavage (BAL) techniques in which 3 × 50 ml isotonic saline fluid is administered and recovered from the peripheral airways are mainly used for the diagnosis of infectious and interstitial lung disease [18], in rare cases, however, adenocarcinomas can be diagnosed with this technique, as well. Finally, sputum specimens can, in principal, be screened for the presence of tumor cells. The likelihood for a positive tumor diagnosis and the yield of tumor cells, however, is lowest for sputum material, followed by BAL, brush cytology specimens, and fine-needle material [19]. Sputum as diagnostic specimen, therefore, cannot be recommended [20].
For an optimal yield of material it is recommendable to combine different technical approaches, e.g., brush cytology and biopsy for centrally located tumors [21, 22]. In addition, several needle passages (at least 3–4 per lesion) do increase the likelihood to obtain diagnostically adequate material [23].
Taken together, clearly the type of method applied for specimen retrieval not only influences the ability to render a precise morphological diagnosis, but also impacts on the ability to perform immunohistochemistry and in situ hybridizations (FISH, CISH) on the material and also severely impacts on the yield of RNA and DNA from the respective specimens (see below).
3 Aspects of Tissue Fixation
Regardless whether the specimens submitted to a pathology lab consist of cytology, biopsy, or resection material, one must be aware that besides histomorphology, which requires an immediate and thorough fixation of the specimens, application of additional diagnostic methods is potentially required to obtain a final diagnosis. Since almost all methodological approaches established in routine diagnostics can be performed using formalin-fixed paraffin-embedded tissue (FFPE), separate biobanking of fresh or cryopreserved tissue is usually not required in the majority of cases, but must be considered in specific clinical constellations (e.g., when bacterial cultures are needed) as well as in rare cases where an exploratory scientific approach (e.g., exome sequencing) is intended. Such approaches, however, might become increasingly popular especially when established treatment methods fail and exploratory targeted therapies are a last option.
FFPE tissue is suitable for immunohistochemistry (IHC), DNA extraction, RNA extraction, and FISH/CISH [24]; furthermore, it might be used for electron microscopy after re-embedding. However, when electron microscopy is required in the first place, initial fixation of parts of the specimens with glutaraldehyde is recommended.
Pathological specimens are usually transferred directly into 4 % neutrally buffered formalin and stored until further processing. Time to fixation is critical for the preservation of architecture, antigenicity, and specifically for the integrity of DNA and RNA. Since most of the cases in lung tumor diagnostics consist of biopsy specimens, this is usually not critical, because tiny tissue fragments can directly be transferred to formalin and the penetration of formalin into the probe is almost immediate. However, with larger resection specimens these issues become increasingly critical; this is discussed in more detail in other chapters of this book. Another critical issue might be the time the probe is retained in the fixative solution, before further processing is possible. However, again this applies mainly for resection specimens, since biopsy probes are usually processed more rapidly. Subsequent dehydration and paraffin embedding is usually done with fully automated systems in a highly standardized manner.
Apart from the conventional way of formalin fixation and paraffin embedding, several other ways of tissue preservation do exist. However, none of these approaches has found its way into a broad application in routine diagnostics yet. These methods include cryopreservation by immediate shock freezing of probes in liquid nitrogen but also other novel fixation techniques like HOPE [25], PAXgene® tissue system [26, 27], or RNAlater [28, 29] fixation. All these procedures, however, require either specialized sampling equipment (liquid nitrogen) or tissue handling techniques which are currently not automated and therefore hardly introducible into a routine workflow. Furthermore, all of these methods are more expensive compared to standard FFPE processing.
4 Impact of Preanalytical Variables on Diagnostic Results in Biopsy Specimens
As outlined above, several aspects of preanalytics may strongly influence the outcome of a broad variety of diagnostically necessary analytical methods. This will be discussed in the following in a structured manner for the specific analytical methods applied in the diagnostic setting.
4.1 Morphology
Conventional histomorphology is still the backbone of pathological diagnoses. First and foremost, it is used to confirm the presence of a neoplastic process. In addition, recent data indicate that especially for pulmonary adenocarcinomas (ADC) a precise histomorphological subtyping is of high prognostic and maybe even predictive relevance [30]. Furthermore, it is of utmost importance to (at least) separate tumors with a squamous and a non-squamous phenotype [31], since major druggable driver mutations and amplifications involving, among others, KRAS, EGFR, ALK, BRAF, ROS1, FGFR1, MET, and thus the selection of targeted therapies, are significantly affected by these features and are currently only tested in the specific subentities. Morphology is easily assessed in FFPE material, this is the gold standard preanalytical method in this regard. However, novel fixation techniques like PAXgene® tissue system and HOPE also achieve high morphological standards comparable to that of FFPE procedures (Fig. 1). Cryosections from shock frozen tissue as well as RNAlater fixed tissue show considerably lower quality and require a specialized cutting technique; routine diagnostics cannot be done on these specimens. Therefore, if molecular methods which require frozen tissue are necessary, extra cryoconserved material must be taken in addition to the material necessary for conventional diagnostic purposes. In Fig. 1 morphology examples for differing tissue preserving methods are shown.
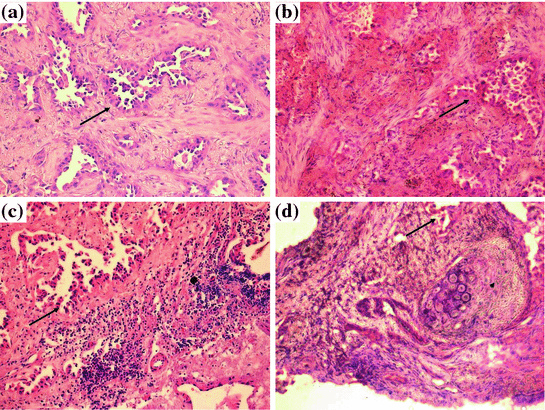

Fig. 1
Morphologic tissue quality in dependence of fixation procedure, a FFPE tissue with well-preserved morphology. b In cryosections morphology is slightly worse; however, the respective structures are still discernable. c HOPE fixation preserves morphology comparable to FFPE procedures. d Tissue fixed in RNAlater shows a somewhat compromised morphology. Arrows neoplastic glands, Arrowhead cartilage
4.2 Immunohistochemistry and FISH
Since in up to 30 % of the NSCLC biopsies a precise subtyping requires additional IHC using specific markers for a squamous (p40, p63, CK5/6), an adenocarcinomatous (TTF-1, napsin, CK7) or even a neuroendocrine differentiation (chromogranin A, synaptophysin, CD56, Ki67) immunohistochemistry is critical for reliable diagnoses [31]. In addition, several IHC-based biomarkers which predict response to targeted agents are currently under development, this specifically includes MET and ALK [32]. For almost all diagnostically relevant antibodies, FFPE tissue is suitable. Since IHC staining is always interpreted in the context of morphology, optimal tissue fixation with high preservation of structural details is important. Poor tissue fixation further results in a loss of immunoreactivity. Tissue fixation depends on time to fixation and several other variables which are not that critical in the lung biopsy setting (see above) but play an important role in the context of resection specimens in general (see other chapters in this book). Other tissue embedding methods including PAXgene® tissue system and HOPE do preserve both morphology and antigenicity; therefore, IHC is easily possible in this context. However, protocols and antibodies must be adjusted and cannot be transferred directly from the FFPE situation.
FISH analytics are necessary for amplification or translocation detection of predictive biomarkers (ROS1, ALK, RET). For FISH analyses, the requirements are comparable to IHC. Besides careful processing of the tissue, the most important thing is proper fixation to maintain the cellular structure and to avoid DNA degradation. From the technical viewpoint, FISH can be theoretically done with most types of fixatives (frozen, FFPE, PAXgene® tissue system, HOPE); however, data on this for some procedures are very sparse and protocols vary considerably.
4.3 Nucleic Acid Extraction
Quality and quantity of nucleic acid extracts are of utmost importance for all subsequent nucleic acid based molecular methods applied in routine diagnostics and translational research. First and foremost, tumor tissue must be marked on stained tissue slides and macro- or microdissected from the very same slide or subsequent unstained slides. Tumor cell content of the microdissected area should be documented, since it influences many factors of the analytics results like, e.g., allele frequencies in sequencing. Prior to tumor dissection the specimens need to be analyzed for a potential tumor heterogeneity including the amount of vital tumor, stroma, necrosis, or potentially contaminating normal lung or inflammatory cells in order to obtain tumor-specific, high-quality nucleic acids. Tumor cell content may vary considerably between biopsies.
Yield and quality of RNA and DNA strongly depends on the type of material used, fixation method, and extraction technology. RNA quality is frequently measured as RNA integrity number (RIN) by using capillary electrophoresis techniques (Agilent bioanalyzer) [33]. Results usually range between 2 (low quality) and 10 (optimum). RIN numbers better than 8 are widely considered as good quality. This RNA quality might serve as a template for most applications including microarray analyses.
The influence of biopsy techniques on RNA/DNA quality is low, when tissue specimens are processed/fixed quickly (1 min. range). Best results are obtained with cryobiopsies followed by forceps biopsies and core needle biopsies. DNA/RNA quality is excellent in most cases of cryobiopsies and suitable for all kinds of molecular analyses including next-generation sequencing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree