Author
Year
Location
TTA before QI process (Min)
TTA after QI process (Min)
Percent decrease
Amado [7]
2011
PICU
164
55
66%
Pakakasam [14]
2011
ED
180
75
58%
Burry [10]
2012
ED
216
Not stated
–
Volpe [8]
2012
ED
99
49
51%
Dobrasz [13]
2013
ED1
103
44
57%
Dobrasz [13]
2013
ED2
141
61
57%
Cash [11]
2014
ED
154
95
38%
Vedi [15]
2014
ED1
148
76
49%
Vedi [15]
2014
ED2
221
65
71%
Cohen [12]
2015
ED
97
64
34%
Salstrom [6]
2015
Hem/Onc Clinic
134
54
60%
Jobson [9]
2015
ED
65
30
54%
Dandoy [17]
2016
ED
137
<50
>63%
Green [16]
2016
Inpatient
99
50
49%
Table 15.2
Quality improvement techniques to reduce time to antibiotics in febrile neutropenic pediatric cancer patients
Clinical practice guidelines or management algorithm |
Multidisciplinary involvement in process design |
Data sharing with key stakeholders |
Staff and patient/parent/caregiver education |
Process improvement methodology such as Lean |
“Sign and hold” orders |
Release of auto-diff results without manual confirmation |
Rapid identification and triage of at-risk patients |
Availability of antibiotics near the patient treatment areas |
Patient/parent/caregiver application of topical anesthetic cream prior to arrival in clinic |
Documentation and discussion of an inpatient patient-specific fever plan |
Time to Antibiotics in Other Pediatric Hematology/Oncology Populations
The incidence of bacteremia in febrile children with sickle cell disease has been reported to be as high as 3–5% [18]. The rate may be lower now due to vaccination against Streptococcus pneumoniae and Haemophilus influenzae and the use of prophylactic penicillin in young children with sickle cell disease. Still, bacteremia is still a major concern in this population [18, 19]. While to our knowledge, there are no studies that look at time to antibiotic versus clinical outcome in febrile sickle cell disease patients, administration of parenteral broad spectrum antibiotics in less than 60 min to febrile sickle cell disease patients has been identified as a quality indicator of high importance for this disorder [20]. Quality improvement studies for time to antibiotics are lacking in this population but have been done for time to pain medication [21, 22].
Using a series of interventions in a population of febrile pediatric patients with central venous catheters in a pediatric academic emergency department, Jobson et al. increased the percentage who had a TTA <60 min from 66 to 99%, sustained for over 2 years, and decreased the mean TTA from a mean of 65 to 30 min. Of note, a baseline racial disparity in the TTA disappeared after these interventions. Key components identified included standardized processes, patient identification cards, and communication of the data with providers and staff [9].
Sepsis in the Hematology/Oncology Patient
Defining Sepsis
As mentioned previously, there is a population of hematology/oncology patients, specifically those with neutropenia, functional or anatomic asplenia, or central venous catheters who are at increased risk of life-threatening infections. Before addressing how to identify and treat these patients, there must first be an understanding of the definitions associated with life-threating infection. At the 2005 International Pediatric Sepsis Consensus Conference, definitions [23] (see Fig. 15.1) were created for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock in the pediatric population. SIRS requires the presence of at least two of the following: fever/hypothermia, tachypnea/respiratory failure, leukopenia/bandemia, and tachycardia/bradycardia. Sepsis is defined as the presence of a suspected or confirmed infection combined with SIRS. Severe sepsis includes sepsis with either cardiovascular dysfunction, respiratory distress syndrome, or dysfunction of at least two other organ systems. Figure 15.2 describes the definition of organ dysfunction in the pediatric population [23]. Finally, septic shock is defined as persistent hypotension despite fluid resuscitation or evidence of tissue hypoperfusion (e.g., altered mental status, decreased urinary output) [23] (Fig. 15.1).


Fig. 15.1
Definitions of systemic inflammatory response syndrome (SIRS), infection, sepsis, severe sepsis, and septic shock. Reprinted with permission from [23]
Early Goal-Directed Therapy
Early goal-directed therapy (EGDT) has been of primary focus from the Surviving Sepsis Campaign, an international collaborative that created guidelines for management of severe sepsis and septic shock revised in 2012 and again in 2016. Principles of EGDT include providing oxygen, aggressive fluid resuscitation, early antibiotic administration, inotropic support for fluid-resistant shock, and steroid administration for inotropic resistant shock [24]. The newest guidelines used large validated adult data to change the guidelines emphasizing infection and dysfunction of two organ systems as the best indicators of sepsis [25]. While the Surviving Sepsis Campaign guidelines have been used as a gold standard for sepsis evaluation and treatment, the definitions are not pediatric specific, and the Goldstein criteria [23] remain the most frequently cited pediatric sepsis definitions. In 2010, the addition of pediatric recommendations were released by the American Heart Association as part of Pediatric Advanced Life Support [26] and further revised in 2015 [27, 28] (see Table 15.4).
Table 15.4
Pediatric advanced life support septic shock (SS) guidelines
Timely recognition of septic shock | Initiation of interventions/frequent reassessment |
|---|---|
Placement of PIV | Within 5 min of recognition |
20 cc/kg isotonic crystalloid fluid | Within 5 min of recognition then reassess |
Antibiotic administration | Within 60 min of recognition |
Vasoactive agent administration | Within 60 min of recognition |
When focusing on pediatric specific literature, studies have reported a noteworthy increase over the past decade in the number of sepsis cases identified in pediatric hospitals [29]. Yet, the rate of sepsis in the pediatric population differs from study to study likely due to a myriad of factors including different patient populations, study design, reporting bias, detection bias, differing sets of diagnostic criteria, and differing sources of data [30].
Early identification of the febrile patient who is likely neutropenic, functionally or anatomically asplenic, has an indwelling central venous line, or is immunosuppressed is critical to assess for SIRS, sepsis and septic shock in the hematology/oncology population. Once suspicion of sepsis is identified, initial management should include EGDT with concentration on frequent assessment [26–28]. Intravenous access must be quickly established followed by a 20 mL/kg bolus with isotonic fluid and timely reassessment for tissue hypoperfusion [28]. Additional fluid boluses should be considered based on these frequent assessments [27]. Compared with adults, children can remain in compensated shock with persistent tachycardia. They may also present later with hypotension as a sign of irreversible cardiovascular collapse [26]. The pediatric consensus guidelines are designed to identify patients with compensated septic shock allowing for early intervention to prevent cases of profound decompensation [23].
During fluid resuscitation, the clinician should also remain aware of the patient’s respiratory status. The Fluid Expansion as Supportive Therapy [31] trial looked at treatment of children with a febrile illness complicated by impaired consciousness, respiratory distress or both, and impaired perfusion. The trial concluded that early fluid therapy increased mortality [31]. However, further review of subsequent literature analyzing the FEAST trial was included in the Pediatric Advanced Life Support: 2015 International Consensus on Cardiopulmonary Resuscitation which does not recommend limiting resuscitation fluids for children in septic shock but does recommend utilizing caution if the resuscitation occurs in a place with extremely limited critical care resources [27, 28].
The other principles of EGDT: providing oxygen, early antibiotic administration, and inotropic support for fluid-resistant shock [24], also should each be addressed as soon as signs of SIRS/sepsis/septic shock are recognized. As oxygen delivery is dependent on cardiac output and the oxygen-carrying capacity of blood, oxygen delivery can be increased by first providing 100% inspired oxygen and additionally by volume expansion with rapid infusion of isotonic fluid or packed red blood cells when indicated by hemoglobin and hematocrit results. The administration of intravenous antibiotics must be administered within the first hour of suspected sepsis or septic shock [32].
Performance Improvement
Poor patient outcomes from sepsis can be mitigated with early identification of sepsis and subsequent timely initiation of proven therapies [33]. The Surviving Sepsis Campaign 2012 recommendations include the utilization of performance improvement methods to improve patient outcomes [32]. The original campaign implemented a set of goals in the form of a bundle in multiple hospital settings and demonstrated not only improved quality of treatment of sepsis but additionally decreased mortality [33] (see Table 15.3).
Table 15.3
Surviving sepsis campaign bundles
To be completed within 3 h: |
(1) Measure lactate level |
(2) Obtain blood cultures prior to administration of antibiotics |
(3) Administer broad spectrum antibiotics |
(4) Administer 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L |
To be completed within 6 h: |
(5) Apply vasopressors (for hypotension that does not respond to initial fluid resuscitation) |
to maintain a mean arterial pressure (MAP) ≤ 65 mm Hg |
(6) In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate 4 mmol/L (36 mg/dL): - Measure central venous pressure (CVP)a - Measure central venous oxygen saturation (ScvO2)a |
(7) Remeasure lactate if initial lactate was elevateda |
Implementation of standardized sepsis protocols in pediatrics foster improved recognition of septic patients [34, 35] and more timely delivery of critical interventions [34, 36] in both inpatient and emergency department settings. Early identification of patients who may require EGDT for possible sepsis can be achieved by training staff on age-appropriate vital signs and abnormal variation in vital signs based on temperature elevation [34–36]. Additional training must focus on compliance with current treatment guidelines. Paul et al. were able to increase compliance with PALS sepsis guidelines with the use of QI methodology [36]. As pediatric institutions continue to undertake development of plans for adapting and sustaining sepsis protocols, collaboration and continued quality improvement will remain vital to reaching the goal of enhancing outcomes. In addition to institutional quality improvement efforts, national and international efforts may offer the opportunity for more rapid learning through shared quality improvement collaboration as done in the past by the Surviving Sepsis Campaign [32] and currently by the Children’s Hospital Association Improving Sepsis Outcomes Collaborative [37].
Safe Handoffs in Pediatric Hematology/Oncology
All care providers must transition the care of their patients to an oncoming provider at the end of a clinical shift. Hematology/oncology patients frequently have complicated conditions; thus, it is important for providers to be competent in patient handovers.
A successful handoff is defined by The Joint Commission’s Center for Transforming Healthcare as “a transfer and acceptance of responsibility for patient care that is achieved through effective communication. It is a real time process of passing patient-specific information from one caregiver to another or from one team of caregivers to another to ensure the continuity and safety of that patient’s care [38]. In 2006, The Joint Commission identified handoffs as a major risk to patient safety. In response one of its national patient safety goals was for hospitals “to implement a standardized approach to “hand off” communications, including an opportunity to ask and respond to questions.” [39, 40] When a provider relinquishes the care of a hematology/oncology patient to another provider, the effective transfer of information becomes critical to patient safety, and the loss of critical information can lead to medical error and adverse events [41].
Increased attention to handoffs occurred when the American Council for Graduate Medical Education (ACGME) restricted resident work hours to 80 h per week in 2003 and then added additional restrictions in 2011 [42, 43]. This resulted in a significant increase in the number of patient transitions of care. The increased number of patient handoffs subsequently led to delayed care [44], delayed disposition [45], redundancy in work [46], and uncertainty regarding future care [47].
Currently, the ACGME requires residents to be competent at handing over patients [48]. Yet, initial exploration of resident ability to transition patients demonstrated that interns often overestimate the effectiveness of their handoffs. Additionally, in one study residents reported that a patient was harmed 50% of the time on their previous rotation due to a handoff error [49]. To achieve handoff consistency among residents in an academic institution, it has been suggested that standardization of handoffs and a content checklist are crucial to appropriate communication [50].
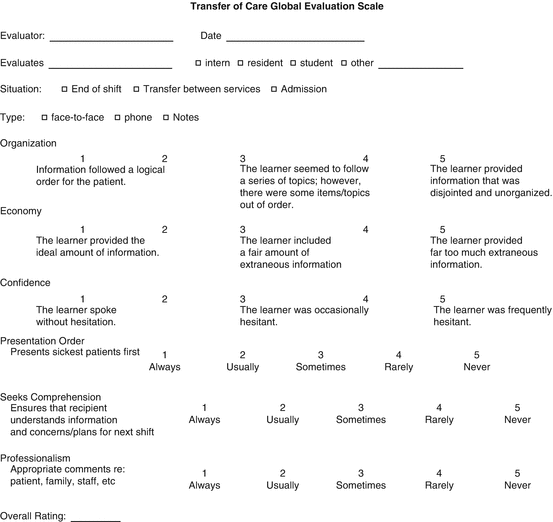
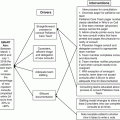
As research progressed in the field of handoffs, communication was noted to be an essential part of an effective handoff (1, Joint Commission 2007). Poor communication has been cited by The Joint Commission as the root cause of up to two-thirds of sentinel events [51]. In addition, it has been estimated that up to one quarter of all malpractice claims are a direct result of communication errors [52]. To improve communication, one suggested strategy is the utilization of mnemonics which can provide structure and act as a memory aid [53]. In pediatrics, the I-Pass mnemonic [54] (Fig. 15.3) and the use of checklists [50] have established improvement in handoffs. The I-Pass mnemonic, when implemented as part of a handoff bundle to include standardized communication, training, as well as efforts to minimize distraction, ultimately revealed decreased rates of medical errors [41]. Measurement of improvement in communication can be done using a Likert scale to score elements of communication such as confidence, organization, and included/excluded content [50] (Fig. 15.4). Additionally, for improved handoff communication, one should strive to communicate face to face which has proven to be the superior method of communication when compared with phone, email, and paper communication [55].
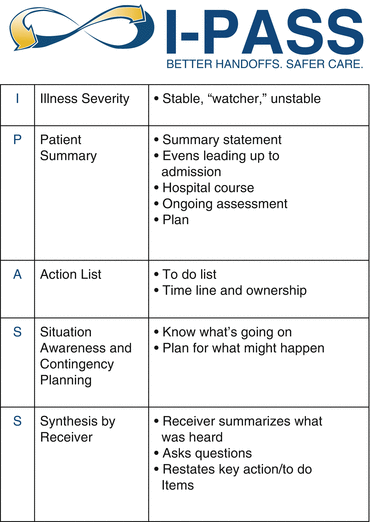

Fig. 15.3
Elements of the I-pass handoff tool. Reprinted from [54] with permission from the I-PASS Study Group
Two additional factors that can weaken handoff quality are lack of formal training [39] and lack of standardized tools to assess handoffs [53, 56] Thus, whether a health system ultimately chooses to utilize a checklist or a mnemonic for care transition, it is imperative that all providers receive standardized training on the tool. By providing standardization, providers obtain structure so that the “rules” of interaction (e.g., content and order) do not need to be negotiated; if no information is given, it implies there was nothing that required mention, and information is conveyed more efficiently and reliably [56]. One must also develop an assessment tool for ongoing evaluation and improvement [53]. For example, a global assessment tool (Fig. 15.4) encourages evaluation by direct observation [50].
Once the health system has determined the tool, education, and assessment that will be implemented, there are some general strategies that will be undertaken to make handoffs as successful as possible. These include being clear, concise, and organized and asking the receiver if they have any questions after each patient; being certain to impart critical data including pertinent positives and negatives about the patient; allowing the appropriate amount of time to relay information; and doing it where interruptions are minimized. For instance, implement a page-free zone or a designated quiet space in which to handover patients. Identify and present the most critical patients first when the receivers’ attention is at its best. Finally, the receiver should be empowered to not accept a poor patient handoff and to ask clarifying questions.
Influenza Vaccination in Pediatric Hematology/Oncology Practices
Influenza, a common upper respiratory tract infection, can cause major complications, especially in the immune compromised host [57, 58]. Complications include respiratory failure, secondary pneumonia and bacteremia, and prolonged viral shedding. Cancer treatment delays are common [59]. Therefore, preventing this infection in children with sickle cell disease or cancer is of high importance. The percentage of patients in each of these groups who did receive the appropriate influenza vaccine in the recommended time frame for each year is a good process measure for influenza prevention.
Influenza Vaccination in Pediatric Cancer Patients
Pediatric cancer patients are at increased risk for severe influenza-related illness. Two studies in the recent era assessed influenza complications in hospitalized pediatric oncology patients. In a 5-year period with 27 clinical encounters due to influenza, 63% of which were treated with antiviral medication, Tasian et al. [59] found that 15% required mechanical ventilation, 22% required oxygen support, 15% developed bacteremia, and 11% had hospitalizations in excess of 30 day. The influenza vaccination status of these patients was not reported. In a similar 5-year period, Kersun et al. [60] describe 39 patients, 46% of which had received immunization. Of these, 20% had respiratory complications, 10% intensive care admissions, and 5% died.
Treatment with antiviral agents such as oseltamivir or zanamivir for specific strains of influenza and in appropriately aged patients can decrease the severity of infection and complication rate for pediatric cancer patients infected with influenza [58]. However, because complications exist even in treated patients, the potential for cancer treatment interruption, and possible exposure of other at-risk patients to the illness, prevention is a better strategy. As infection of patients can occur from healthcare personnel, universal vaccination of pediatric hematology/oncology providers and staff should be performed [58].
Influenza Vaccination Safety and Efficacy in Pediatric Cancer Patients
Overall, patients receiving chemotherapy have a decreased serologic response to influenza vaccine when compared to healthy children. Patients with AML or within 1 year of stem-cell transplant have lower response rates [58]. Mavinkurve-Groothuis et al. [61] found that 92% of patients with a normal absolute lymphocyte count for age had a protective immune response to H1N1 vaccine as opposed to 33% with a low absolute lymphocyte count. They did not find a difference between hematologic malignancy (mostly ALL) and solid tumor patients in response to H1N1 vaccine. None of their patients with an absolute T-cell count below 200 per microliter achieved protective levels. Older age or perhaps the larger dose administered to older children may predict a higher vaccine response rate in children with ALL [62, 63]. It is not clear that the serologic titer associated with a protective effect in healthy individuals is applicable to pediatric oncology patients [58, 63]. Children who have completed therapy for cancer, with the exception of stem-cell transplant patients, have a better response to immunization [63].
The adverse reaction rate to inactivated influenza vaccine is not higher in pediatric cancer patients than in the general population [58, 63]. Thus, despite lower serologic response rates for children on chemotherapy compared to the general pediatric population, that a significant proportion of on-therapy patients do respond, that off-therapy patients respond well, the low adverse reaction rate and the significant morbidity of influenza in this population give strong support to the recommendation that this population be vaccinated against this infection with the inactivated form of the vaccine.
Influenza Vaccination Rates in Pediatric Oncology Patients
Despite the above reasons for vaccinating pediatric cancer patients against influenza, vaccination rates in this population have been disappointing [58, 60]. Some of the stated barriers to adequate vaccination are noted in Table 15.5. Adult survivors of childhood cancer who are also at high risk for influenza-related complications due to late effects of their cancer treatments also have a low rate of vaccination [64].
Table 15.5
Barriers to influenza vaccination in pediatric cancer patients
Parental concern about vaccine side effects |
Parental fear that vaccination will cause influenza illness |
Provider belief that vaccination is ineffective |
System failure to identify unimmunized children |
System failure to administer vaccine to eligible children |
Freedman et al. [65] used five interventions to increase influenza vaccination rates in their pediatric oncology program: (a) parent/family education, (b) use of the electronic health record to identify vaccine-eligible patients, (c) brightly-colored identification bracelets attached to vaccine-eligible patients in the ambulatory environment, (d) inclusion of influenza vaccine in the discharge order set, and (e) provider education. As a result, when compared to the prior year’s population, there was a significant increase in the percentage of fully immunized patients (64.5% vs. 44.4%, p = 0.001) and a proportionate decrease in unimmunized patients (45.2% vs. 22.5%, p = 0.001). The percentage of patients who were partially immunized did not change significantly (13.0% vs. 10.4%, p = 0.19). They demonstrate, as with most quality improvement projects to improve adherence in pediatrics, multidisciplinary and patient/parent/family education, and systemic factors need to be addressed.
Influenza Vaccination in Pediatric Sickle Cell Disease
Sickle cell disease (SCD) patients are also at increased risk for adverse outcomes from influenza infection [57]. The hospitalization rate for influenza illness in children with sickle cell disease is 56 times that of children without SCD and even higher for children identified as having homozygous HbSS disease [66]. Influenza can cause acute chest syndrome [67].
Influenza vaccine is both safe and effective in SCD. Unlike pediatric cancer patients, the large majority of children with SCD achieve protective antibody titers in response to inactivated influenza vaccine [68–71]. Purohit et al. [68] did report a decreased response to inactivated H1N1 in SCD patients on chronic transfusion. Hydroxyurea use and splenectomy did not appear to impact response [68, 69]. The vaccine is well tolerated in this population [69–71]. The inactivated trivalent influenza vaccine does not appear to increase the rate of hospitalization for vaso-occlusive crisis within 2 weeks of administration [72]. Because of the morbidity of influenza in SCD patients and the proven efficacy and safety of the inactivated vaccine, the CDC, AAP, and WHO all recommend influenza vaccination for sickle cell disease [57]. An annual influenza vaccine is a recommended quality measure for sickle cell disease [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree