Highlights
- •
High grade and very high grade NMIBC has the potential to reoccur and progress and the BCG intravesical therapy is an adjuvant gold standard treatment.
- •
Smoking impacts appearance and oncological outcomes of NMIBC, even in BCG treated patients.
- •
In the EAU21 risk group classification the high-risk patients with heavy long-term smoking exposure showed a higher risk of progression when compared to the high-risk group with poorer survival outcomes.
Abstract
Introduction
The nonmuscle invasive bladder cancer treated with BCG instillations in patients who smoke could potentially lead to poorer oncological results in the light of the new EAU risk groups classification for NMIBC that did not include BCG treated patients or smoking status.
Patient and Methods
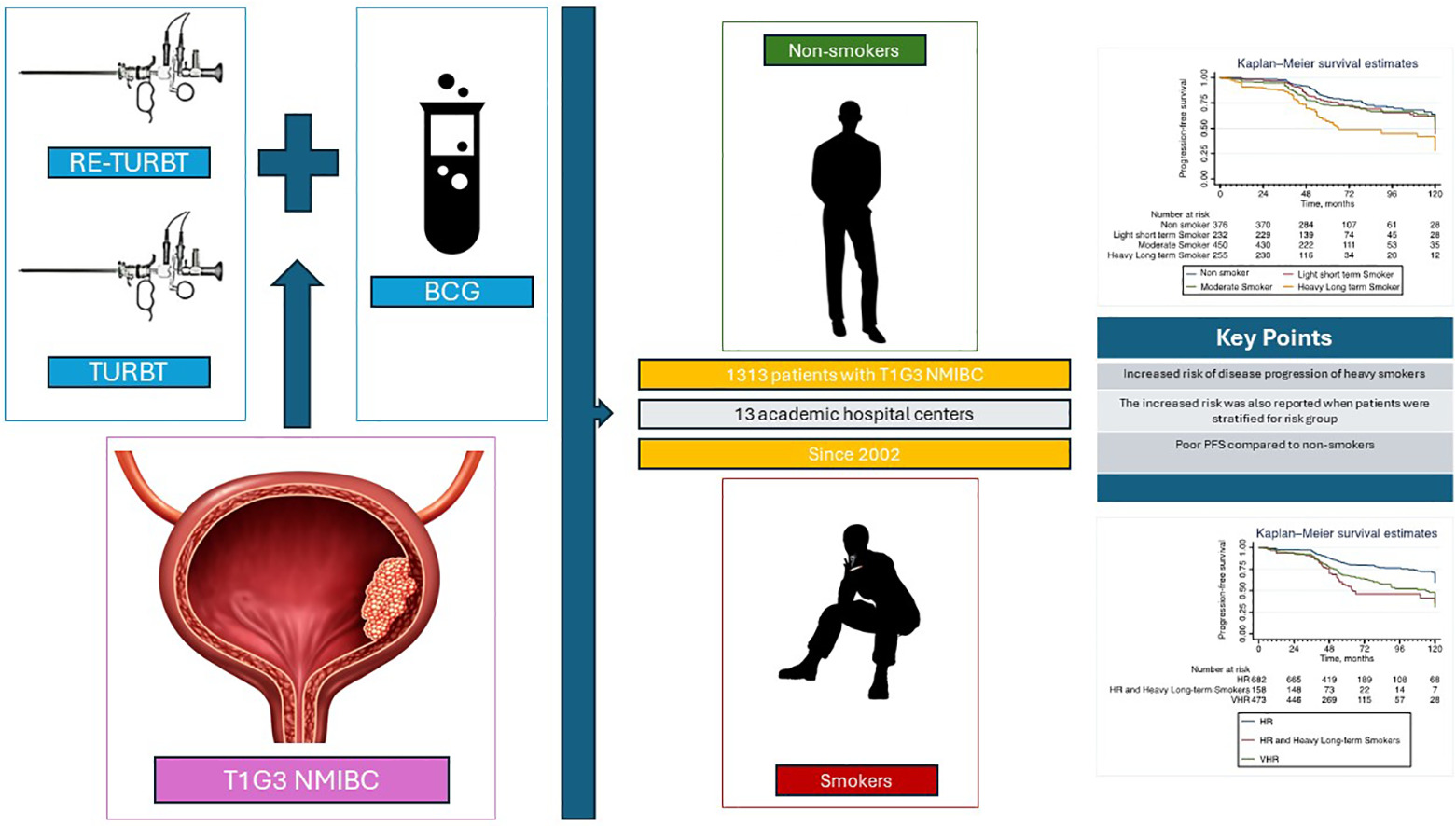
Outcomes from 1313 patients with nonmuscle invasive bladder cancer treated with TURBT, re-TURBT and BCG instillations at 13 academic hospital centers, since 2002, has been included in this retrospective study. The study variables, including cumulative smoking exposure have been analyzed. A multivariable Cox proportional hazard model was used to assess associations between smoking variables and disease progression and repeated in the EAU high risk and very high-risk group. The statistical significance threshold was set at 0.05, and the statistical analysis was performed using Stata/SE version 17 (StataCorp, College Station, TX, USA).
Results
Cox regression analysis revealed in 1313 patients diagnosed with T1G3 NMIBC that patients with a history of heavy and long-term smoking faced a more than twofold increased risk of disease progression compared to nonsmoker patients (HR 2.35; 95% CI: 1.7-3.2; P < 0.01) and a significantly poorer PFS for patients with a history of heavy long-term smoke exposure ( P < 0.01). Patients with heavy long-term smoking exposure according to the EAU21 high-risk group had a PFS comparable to very high-risk patients and high-risk patients with heavy long-term smoking exposure showed a higher risk of progression when compared to the high-risk group (HR 1.4; 95% CI: 1.3-1.6; P < 0.01).
Conclusions
This study adds valuable information on the relationship between smoking and the progression of NMIBC and BCG therapy. The findings emphasize the need for healthcare providers to consider a patient’s smoking history when managing NMIBC and express the need for individualized smoking cessation counseling and individualized treatment approach.
Graphical abstract
1
Introduction
Nonmuscle invasive bladder cancer (NMIBC) is a complex and clinically challenging malignancy, known for its high recurrence and progression rates [ ]. Over the years, a big effort has been made to enhance our understanding of factors impacting NMIBC outcomes and to identify variables that affect patient prognosis [ ]. Among these factors, the role of smoking and smoking habits is pivotal [ ].
Smoking, a well-established public health concern, has long been associated with numerous adverse health outcomes, including various types of cancer. In the case of bladder cancer (BC), multiple studies consistently reveal a strong link between smoking and disease development [ ]. Carcinogens present in cigarette smoke, such as aromatic amines and polycyclic aromatic hydrocarbons, accumulate in the bladder, causing direct damage to the urothelial lining and potentially triggering cancerous growth [ ].
However, smoking’s influence extends beyond initiating BC; it significantly impacts the disease’s natural history, making patient’ smoking status assessment a crucial consideration in the context of NMIBC treatment and follow-up [ ].
Intravesical instillation with Bacillus Calmette Guerin (BCG) serves as the gold standard adjuvant therapy in high-risk NMIBC patients following Transurethral Resection of the Bladder Tumor (TURBT) to reduce the risk of recurrence [ ]. BCG’s mechanism of action involves immune system stimulation, triggering an inflammatory response [ ]. This response includes the release of pro-inflammatory cytokines that attract cytotoxic immune cells such as neutrophils, monocytes, CD4+ and CD8+ T cells, B cells, macrophages, and natural killer cells [ , ].
Some authors have suggested that smoking may diminish the effectiveness of BCG therapy, by modulating cytokine activity and reducing the response of B- and T-cells, the activation of natural killer cells, potentially increasing the risk of disease recurrence and progression [ , ].
Recently, the European Association of Urology (EAU) Guidelines Panel revised the risk groups classification for NMIBC, establishing 4 distinct risk groups (namely, low [LR], intermediate [IR], high [HR], and very high [VHR] risk) based in the risk of progressing to Muscle Invasive Bladder Cancer (MIBC)[ ]. Nevertheless, the newly developed scoring system was formulated by examining data exclusively from patients who did not undergo BCG intravesical therapy [ , ]. In addition, smoking status and habits were not included in this classification.
The objective of this study was to further clarify the effects of smoking status, quantity, duration, and cumulative exposure on disease progression in primary NMIBC patients treated with TURBT and adjuvant intravesical BCG therapy, within the framework of the new EAU NMIBC prognostic factor risk groups.
2
Patients and methods
This retrospective multicenter study included a total of 1313 patients who had undergone TURBT and were diagnosed with T1G3 NMIBC at 13 healthcare centers and research institutions since January 1, 2002. All patients underwent repeat Transurethral Resection (re-TURBT) (within 2-6 weeks), followed by intravesical BCG instillations, according to EAU NMIBC guidelines. The BCG course included a weekly induction regimen for 6 weeks, followed by 3 weekly instillations at 3 and 6 months, and subsequently every 6 months for up to 3 years, according to the so-called Southwest Oncology Group (SWOG) scheme [ ]. Tumor grade was assessed in accordance with the WHO 1973 grading system. Cystoscopy surveillance schedules were standardized across all providers, adhering to available NMIBC guidelines [ ].
The study variables collected within our dataset encompassed patient demographics, tumor stage, grade, size, and multifocality, along with the presence of concurrent Carcinoma in Situ (CIS) and Lymphovascular Invasion (LVI). Additional variables related to smoking history included smoking status (never smoked, former smoker, or active smoker), smoking duration (in years), pack-year count, and the number of cigarettes smoked per day (CPD) defined as an estimate of the average during the years the patient smoked. Cumulative smoking exposure was categorized, according to prior studies, as light (≤19 CPD & ≤19 years of smoking), or heavy (>19 CPD and >19 years of smoking), with all other cases considered as moderate exposure [ ].
The primary endpoint of this study was to assess the association between smoking and disease progression, defined as the diagnosis of MIBC or distant metastasis during follow-up. All survival outcomes were measured from the date of the first TURBT; patients were censored at the last follow-up when they were known to be free from the event or at the time of death.
Subsequently, we conducted an exploratory analysis, considering individuals classified as Heavy Long-term Smokers and defined HR as per the EAU21 guidelines in the VHR group.
Descriptive statistics were employed to summarize the study cohort. Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test, while continuous variables were tested using the Wilcoxon rank-sum test. The median follow-up duration for the study was determined using the reverse Kaplan-Meier method. Survival estimates were calculated using the Kaplan-Meier method. A multivariable Cox proportional hazard model was used to assess associations between smoking variables and disease progression. The multivariable models were adjusted for the following covariates: multifocality, size > 3cm, concomitant CIS, gender, and age. The analyses were repeated in the EAU high risk (HR) and very high-risk (VHR) group. The statistical significance threshold was set at 0.05, and the statistical analysis was performed using Stata/SE version 17 (StataCorp, College Station, TX, USA).
3
Results
The data from a total of 1313 patients diagnosed with T1G3 NMIBC were included in the analysis.
Detailed clinical and pathological characteristics are presented in Table 1 , The proportion of patients who completed the BCG maintenance therapy regimen in our cohort was 29%. Over a median follow-up period of 50 months (IQR 41-75 months), 344 patients experienced disease progression to MIBC or metastatic disease. Furthermore, 158 patients died of all causes of death, including 65 who specifically died due to bladder cancer.
| Overall Population (N = 1313) | |
|---|---|
| Age at surgery, median (IQR) | 70 (65, 77) |
| Gender, n (%) | |
| Male | 1067 (81) |
| Focality, n (%) | |
| Multifocal | 555 (42) |
| Tumor Size, n (%) | |
| >3 cm | 835 (64) |
| Concomitant CIS, n (%) | |
| Yes | 199 (15) |
| LVI, n (%) | |
| Yes | 209 (16) |
| EAU Risk Group, n (%) | |
| High Risk | 840 (64) |
| Very-High Risk | 473 (36) |
| Smoking status, n (%) | |
| Never | 376 (29) |
| Former | 380 (29) |
| Active | 557 (42) |
| Smoke load, pack-year, median (IQR) | 15 (9, 30) |
| Smoking duration, years, median (IQR) | 25 (15, 35) |
| Cigarettes per day, median (IQR) | 12 (10, 20) |
| Cumulative smoke exposure | |
| Non Smoker | 376 (29) |
| Light short term Smoker | 232 (18) |
| Moderate Smoker | 450 (34) |
| Heavy Long term Smoker | 255 (19) |
Cox regression analysis revealed an increased risk of disease progression for active and former smokers compared to patients who had never smoked (HR: 1.46; 95% CI:1.10–1.94; P = 0.008 and HR: 1.55; 95% CI: 1.14–2.10; P = 0.005) ( Table 2 ).
| All patients | HR | VHR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P -value | HR | 95% CI | P -value | HR | 95% CI | P -value | |
| Smoking status | ||||||||||
| Never | Ref | Ref | Ref | |||||||
| Former | 1.55 | 1.14–2.10 | 0.005 | 1.55 | 1.01–2.35 | 0.041 | 1.49 | 0.94–2.38 | 0.087 | |
| Active | 1.46 | 1.10–1.94 | 0.008 | 1.58 | 1.1–2.27 | 0.013 | 1.25 | 0.79–1.96 | 0.336 | |
| Packs year | 1.01 | 1.01–1.02 | <0.01 | 1.02 | 1.01–1.02 | <0.01 | 1.01 | 1–1.01 | <0.01 | |
| Year of smoking | 1.01 | 1–1.02 | <0.01 | 1.02 | 1.01–1-02 | <0.01 | 1.01 | 0.99–1.02 | 0.086 | |
| Cigarettes per day | 1.02 | 1.01–1.03 | <0.01 | 1.02 | 1.01–1.04 | <0.01 | 1.02 | 1–1.03 | <0.01 | |
| Cumulative Smoke Exposure | ||||||||||
| Non smoker | Ref | Ref | Ref | |||||||
| Light short term Smoker | 1.14 | 0.8–1.6 | 0.452 | 1.10 | 0.68–1.79 | 0.680 | 1.14 | 0.69–1.90 | 0.597 | |
| Moderate Smoker | 1.30 | 0.97–1.7 | 0.078 | 1.34 | 0.90–2 | 0.145 | 1.23 | 0.77–1.96 | 0.368 | |
| Heavy Long term Smoker | 2.35 | 1.7–3.2 | <0.01 | 2.50 | 1.67–3.7 | <0.01 | 2 | 1.20–3.33 | 0.007 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree