Antigen
Location
Expression in tumor
Prevalence (%)
Description
CEA
Cell surface (GPI-linked)
Overexpressed
30–100
Glycoprotein, normally expressed only on oncofetal tissues. Functions as cell-adhesion molecule. First tumor antigen to be described
Her2-neu
Transmembrane
Overexpressed
>50
A receptor tyrosine kinase, member of the EGF−receptor family, involved in cell growth and differentiation
K-Ras
Intracellular
Mutated self
90
Mutated form of ras, a GTPase important for cell proliferation, differentiation, and survival
Mesothelin
Cell surface (GPI-linked)
Overexpressed
~100
GPI-linked glycoprotein normally expressed on the surface of mesothelial cells lining the pleura, peritoneum, and pericardium at low levels. Binding partner of CA125/MUC16
MUC-1
Transmembrane
Overexpressed, hypoglycosylation
90
Type 1 transmembrane glycoprotein, expressed on apical surface of ductal and glandular epithelial cells at low levels. Extracellular domain has a polypeptide core with multiple tandem repeats of 20 amino acids
p53
Intracellular
Mutated self
50–70
Tumor suppressor that regulates cell cycle. Normally inhibits survivin at the transcription level and can initiate apoptosis if DNA damage is unrepairable
Survivin
Intracellular
Overexpressed
80
Member of IAP family. Inhibits caspase activation; is found in most human tumors and fetal tissue, but is completely absent in terminally differentiated cells
Telomerase
Intracellular
Overexpressed
95
Ribonucleoprotein that is responsible for RNA-dependent synthesis of telomeric DNA. TERT is its catalytic subunit
VEGFR2
Transmembrane
Overexpressed
64
A tyrosine kinase and member of platelet-derived growth factor family. Receptor for VEGF with functions in blood vessel development
12.4 Immunotherapies in Pancreatic Cancer
Both active and passive immunity have been tested in pancreatic cancer (PC) to elicit immune responses to tumor cells. Targeting active immunity through vaccines attempts to induce long-term cellular (T-cell) immunity against cancer cells, whereas antibody-based immunotherapy targets PC cells, but does not stimulate long-term immunity. Recent active and passive immunotherapies in PC will be discussed in this section.
12.4.1 Active Immunotherapy
The goal of tumor-specific vaccines is to present tumor-associated antigens (TAA) to immune cells and produce potent and lasting cytotoxic effects against tumor cells. Antigen presenting cells (APC) such as dendritic cells (DCs) and T cells (CD4/CD8) are the targets and effectors of this immune response. Different types of vaccines have been developed and specific examples are reviewed below.
12.4.1.1 Whole Cell Vaccines
Using irradiated tumor cell vaccines can produce potent immune responses to multiple TAAs present on pancreatic tumor cells. Allogeneic or autologous tumor cells can be used to develop vaccines. Advantages of whole cell vaccines include tumor cells can be grown in vitro, specific TAAs do not need to be identified, polyclonal tumor specific T cell populations are generated, and cells can be altered to express surface proteins or secretable factors that induce strong immune responses [53].
One such example is granulocyte-macrophage colony stimulating factor (GM-CSF) secreting tumor cells. Dranoff has previously shown that GM-CSF secreting cells induce long-lasting immunity in melanoma models [54]. A phase I study by Jaffee et al. took 14 patients with PC and inoculated variable doses of GM-CSF secreting allogeneic tumor cells 8 weeks after pancreaticoduodenectomy. Three patients developed delayed-type hypersensitivity responses and remained disease free at 25 months [55].
A phase II, single-institution study treated 60 patients after surgical resection (R0 or R1) with 5 × 108 GM-CSF secreting tumor cells 8–10 weeks after surgery [56]. Patients then received 5 FU chemoradiotherapy and up to four more vaccinations. Median disease-free survival and overall survival were 17.3 and 24.8 months, respectively. The most common side effects were erythema, induration and pain at the vaccination site, and the only grade 3–4 side effect was eosinophilia in two patients (1 %). Disease-free survival was correlated with the induction of mesothelin-specific CD8+ T cells. Mesothelin is a membrane-bound protein that is highly expressed in pancreatic cancer cells, but has low expression in normal tissue, making it a possible target of immune therapy [39] Laheru and colleagues showed that in metastatic cancer, a GM-CSF tumor cell vaccine given with cyclophosphamide also induces a mesothelin-specific CD8+ T-cell response [57].
Cell surface molecule expression can be modified to induce immune responses. The most successful model exploits hyperacute rejection due to alpha-galactosyl (αGal). Nonprimate mammals express αGal, but humans have a nonfunctional gene. Repeated exposure by gut flora to αGal epitopes leads to high expression of anti-αGal antibodies, which constitutes up to 1 % of circulating IgG [58]. The hyperacute antibody-mediated rejection leads to cell-mediated immunity against TAA in murine models of melanoma [59]. A phase II open-label multi-institutional trial evaluated algenpantucel-L with adjuvant gemcitabine and 5-FU-based radiotherapy after surgical resection [60]. Adjuvant therapy was the same as the RTOG-9704 study protocol [61]; 73 patients were enrolled and 69 received 100 million or 300 million cells per injection and a median of 12 vaccinations. The primary endpoint of 1-year disease-free survival was 62 % and secondary endpoint of 1-year overall survival was 86 % with no serious side effects attributed to immunotherapy. Survival with the addition of algenpantucel-L compared favorably with RTOG-9704 study. A phase III multicenter randomized controlled trial to evaluate adjuvant gemcitabine alone or with 5-FU-based radiotherapy with or without algenpantucel-L was completed in January 2014 [62].
12.4.1.2 Peptide Vaccines
Small antigenic protein fragments are used to develop peptide vaccines. These peptides can be produced economically and safely with no risk of infectious material. In addition, no autologous tissue is required. The drawbacks are that they can be poorly immunogenic and adjuvants may be required to induce a meaningful response [63]. Multiple peptide vaccines have been developed to target PC and have shown promising results.
12.4.1.3 KRAS Vaccines
The v-Ki-ras2 Kristin rat sarcoma (KRAS) viral oncogene encodes a GTPase important for signal transduction. Mutations can be found in the majority of pancreatic cancers as well as in lung and colon cancers [36] In 1995, Gjertsen showed that T-cell response could be activated using a Kristin rat sarcoma mutant (codon 12) peptide vaccine [64]. A follow-up phase I/II study exposed autologous APCs to Kristin rat sarcoma peptide vaccine ex vivo, then reinfused the activated APCs in five patients. Two out of five produced immune responses to ras [65]. To improve immunogenicity of the vaccine, GM-CSF was used as an adjuvant to the K-Ras vaccine in 48 patients with PC. The vaccine was well tolerated and patients who showed an immune response had superior survival compared to nonresponders (148 days vs. 61 days) [66]. Unfortunately, a follow-up study showed the safety of k-Ras/GM-CSF vaccine, but did not produce an immune response [67].
Weden and colleagues were able to induce immune responses using Kristin rat sarcoma vaccines prepared with long synthetic peptides. These peptides require processing and presentation by APC and induce polyclonal T cells that have specificity to mutated Kristin rat sarcoma [68]. In 23 patients who were vaccinated after surgical resection (20 evaluable), 4/20 (20 %) were alive at 10 years, whereas 0/87 in a cohort of nonvaccinated patients during that same period were alive [69].
12.4.1.4 VEGF Vaccines
Vascular endothelial growth factor (VEGF) is a key signaling protein in angiogenesis. Overexpression of VEGF is seen in pancreatic cancers and is associated with larger tumor size and enhanced local spread [52]. A phase I study used VEGFR2-169 epitope, a peptide vaccine for VEGF receptor 2, in combination with gemcitabine in advanced PC (unresectable or metastatic disease). Of the 18 patients receiving at least 1 vaccination, 61 % developed cytotoxic T lymphocytes specific to VEGFR2 and median survival was 8.7 months [70]. Recently, the results of a phase II/III randomized placebo controlled study of gemcitabine +/−VEGFR2 vaccine showed no survival advantage [71].
12.4.1.5 Telomerase Vaccines
Telomeres are nucleotide sequence repeats at the ends of chromatid that help maintain chromosomes. Telomerase helps to maintain the telomeres, and is reactivated in 85 % of pancreatic cancers [72]. A phase I/II trial looking at GV1001, a peptide vaccine based on the catalytic subunit of telomerase was tested in 48 patients with unresectable pancreatic cancer who were injected with one of three dose levels. The intermediate dose group (300 nmol) showed a median survival of 8.6 months, significantly higher than other groups, and a 1-year survival of 25 % [73]. Follow-up phase III trial did not show a survival advantage of GV1001 versus gemcitabine, but other phase III trials evaluating combination therapy are ongoing [74].
12.4.1.6 Recombinant Vaccines
To increase antigenicity, viral and bacterial antigens can be added to cancer vaccines to induce a more potent immune response. These infectious antigens can activate the innate immune system, thereby recruiting APC to the site [72]. One example is the TRICOM vaccine [75]. This poxvirus-based vaccination uses B7-1, ICAM-1, and LFA-3 to enhance T-cell stimulation. Kaufman combined CEA and MUC1 antigens with TRICOM expressing vaccinia (PANVAC-V) or fowlpox (PANVAC-F) in a phase I trial. Ten patients with advanced PC were primed with PANVAC-V, and then given three boosters of PANVAC-F monthly up to 12 months. Antibodies to vaccinia were seen in all ten patients and five of eight evaluable patients developed antigen-specific T-cell responses. Median overall survival was 6.3 months and significant prolonged survival was seen in patients who developed anti-CEA and anti-MUC1 immune responses (15.1 months versus 3.9 months) [76].
Listeria vaccines have also been combined with TAA in cancer vaccines. A trial of 28 patients looked at patients with hepatic metastases from four primary tumors (pancreatic, mesothelioma, ovarian, and nonsmall-cell lung cancer). Patients were administered live attenuated listeria vaccine expressing mesothelin, which is expressed on the cell surface of the tumors. An overall of 37 % of subjects lived >15 months with minimal adverse events; half of these patients had PC [77].
12.4.1.7 DNA Vaccines
Vaccines using DNA encoding TAAs have also shown some efficacy in murine models of PC. MUC-1 DNA vaccine was injected in mice along with pancreatic cancer cells expressing MUC-1 (panc02-MUC1) or no MUC-1 (panc02). Vaccinated mice developed cytotoxic T-cell responses to MUC-1 and tumor shrinkage and improved survival was seen in mice with panc02-MUC1 cells. Mice injected with panc02 cells did not show any therapeutic benefit [78].
Survivin has also been tested as a DNA vaccine. A member of the inhibitor apoptosis family, survivin expression is found in PC cells, but not normal pancreatic tissue [48]. Zhu and colleagues inoculated mice with a survivin DNA vaccine or control vector followed by panc02 cells. Survivin inoculated mice showed increased lymphocyte infiltration of tumors compared with control mice. Increased survival and decreased tumor size was also seen in the Survivin group [79]. These findings need to be tested further in human subjects.
12.4.1.8 Antigen-Pulsed Dendritic Cells
Antigen presenting cells (APC) acquire and process antigens to present to T-cells on MHC class I and II molecules. Dendritic cells (DC) are the most important APC, often referred to as “professional APC.” Multiple DC vaccines have been developed and sipuleucel-T is known as the most successful that improved overall survival in castration-resistant prostate cancer versus placebo in a phase III trial [80]. Multiple DC vaccines have been tested in pancreatic cancer with encouraging results. A phase I/II study took autologous DC pulsed with MUC1 peptide and treated 12 patients with resected pancreatic and biliary tumors. No significant toxicity was seen and four patients were alive at 4 years [81]. MUC1-pulsed DC have also been tested in advanced stage PC in a recent pilot study [82]. The vaccine was well tolerated in all seven enrolled patients with no significant side effects. This phase I study did not show a clinical benefit, but confirmed the safety of the vaccine and needs further study.
Another study looked at DC pulsed with carcinoembryonic antigen (CEA) mRNA. Patients with resected PC following neoadjuvant chemoradiotherapy were given autologous DC vaccine for 6 months. All three patients treated were alive 2.5 years after diagnosis with no evidence of disease [83].
12.4.2 Passive Immunotherapy
12.4.2.1 Antibody-Based Therapies
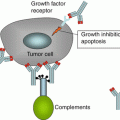
Monoclonal antibodies (mAbs) can affect tumor cells by a number of different mechanisms. Cytotoxic effects including Antibody-dependent cell-mediated cytotoxicity (ADCC), complement-mediated cytotoxicity (CMC), and antibody-dependent cellular phagocytosis leading to apoptosis as well as blockade of cellular receptors-growth factor/cytokine interaction inhibiting growth and survival can be exploited by antibody therapy [84]. Immunoglobulins can also be conjugated to radioisotopes or cytotoxic agents (chemotherapeutic agents, or toxins) and target-specific cellular targets to limit side effects. Numerous studies have evaluated the role of mAb in pancreatic cancer.
Various proteins expressed by PC cells have been targeted by mAb. One such protein expressed by multiple cancer cells is mesothelin. The vast majority of adenocarcinoma of the pancreas express mesothelin, but it is not seen in normal pancreatic tissue or chronic pancreatitis [38]. A phase I study involving 24 patients with mesothelioma, ovarian and pancreatic cancer testing an mAb to mesothelin (MORAb-009) was reported in 2010. MORAb-009 was well tolerated at 200 mg/m2 weekly and 11 patients showed stable disease. One pancreatic cancer patient who progressed on gemcitabine had stable disease for 6 months [85]. A recent phase II study looking at MORAb-009 with gemcitabine versus gemcitabine alone has now been completed and results are pending [112].
Epidermal growth factor receptor (EGFR) is a glycoprotein receptor key in signaling cell proliferation and has been a successful target in numerous cancers including lung, head, and neck cancers. Multiple trials have tested addition of EGFR inhibition to standard chemotherapy in pancreatic cancer. Erlotinib, a small molecule tyrosine kinase inhibitor of EGFR has shown improved survival, but immunotherapy trials have not been as successful [86]. Cetuximab, a chimeric mAb was tested in a large phase III trial run by SWOG comparing gemcitabine with cetuximab versus gemcitabine alone in advanced pancreatic cancer patients. No improvement in progression-free or overall survival was seen with the addition of cetuximab [87]. A fully humanized antibody to EGFR, matuzumab, has also been tested in pancreatic cancer. A phase I study combining matuzumab with gemcitabine was well tolerated and 67 % (8/12 patients) showed partial response or stable disease [88].
Trastuzumab is an mAb that binds epidermal growth factor receptor 2 (HER2), a tyrosine kinase receptor that is part of the EGFR family. It has been effective in both breast and gastric cancers, and is expressed in a significant percent of pancreatic tumors [53]. Treatment with trastuzumab in mouse models has shown efficacy. Nude mice injected with human pancreatic tumor cells showed prolonged survival and decreased metastasis when treated with trastuzumab [89]. Combination therapy has also shown benefit. Larbouret showed that trastuzumab combined with cetuximab resulted in superior survival in mice with human pancreatic cancer xenografts compared to gemcitabine [90]. A phase I/II trial of trastuzumab and cetuximab as second-line therapy in metastatic PC has been completed, but results are not published [91].
Angiogenesis has also been a target of passive immunotherapy, specifically antibodies to vascular endothelial growth factor (VEGF). As noted previously, VEGF and its receptors are often overexpressed in PC [52], and a phase II study showed partial response (21 %) and stable disease (46 %) in metastatic PC patients treated with bevacizumab (anti-VEGF Ab) and gemcitabine [92]. Benefit was not seen in the phase III follow-up study by the Cancer and Leukemia group B (CALGB80303). Overall, 602 patients with advanced PC (85 % metastatic disease) were randomly assigned to gemcitabine+bevacizumab or gemcitabine+placebo. No statistical difference in progression-free or overall survival was seen with the addition of bevacizumab [93]. Bevacizumab has also been tested in combination with gemcitabine and erlotinib in the AVITA study. The addition of bevacizumab increased progression-free survival, but not overall survival [94].
12.5 Radioimmunotherapy
Delivery of radioactive substances by tumor-specific antibodies has produced promising results. Anti-Muc-1 antibody, PAM4, is expressed in 85 % of pancreatic cancers, but not in normal pancreatic tissue [95]. Humanized PAM4 (clivatuzumab tetraxetan) conjugated with yttrium-90, a beta-emitting nucleotide with a radiation path length of 5 mm (90Y-hPAM4), was studied in a phase I trial of 38 untreated patients with advanced PC (86 % stage IV disease). Weekly gemcitabine was used as a radiosensitizer and 90Y-hPAM4 was given weekly starting week 2 on 4 week cycles. Six patients had partial responses (16 %) and 16 (42 % showed stable disease). Median survival was 7.7 months. Grade 3–4 thrombocytopenia or neutropenia developed in 20/38 treated patients after cycle 1. The authors concluded that 90Y-hPAM4 has promising therapeutic activity with manageable side effects [96].
12.6 Immunoconjugates
Antibodies conjugated to cytotoxic agents can concentrate chemotherapy or other toxins at tumor sites and spare normal tissue. CEA has been the target of early immunoconjugate studies in PC. CEA is frequently overexpressed in gastrointestinal tumors including PC. hMN-14 or labetuzumab is an anti-CEA antibody that induces ADCC in vitro in murine colon cancer models [97]. In a pilot study, Labetuzumab was conjugated to SN-38 and infused to mice with human colon and pancreatic cancer xenografts. SN-38 is the active metabolite (two to three times potency) of irinotecan. Improved survival and decreased tumor size was observed in both colon and pancreatic cancer xenografts compared to controls [98].
12.7 Pancreatic Neuroendocrine Immunotherapy
Pancreatic neuroendocrine (pNET) or islet cell tumors comprise 2–3 % of primary pancreatic tumors with increasing incidence over the past 30 years [99, 100]. Early-stage disease is treated with surgical resection, but until recently few options were available for metastatic or unresectable disease. Cytotoxic agents such as streptozocin, dacarbazine, and temozolomide have shown activity, but their application has been limited due to side effects.
Immunotherapy has also been previously studied with interferon. Antitumor effects with interferon alpha include T-cell stimulation as well as cell cycle arrest [101]. Retrospective studies have reported improvement in symptoms and tumor stabilization, but two prospective trials comparing somatostatin analogs with or without interferon therapy did not show any significant difference in tumor response rates or progression-free survival [102, 103]. A large multicenter trial evaluating octreotide with either interferon or bevacizumab is currently enrolling [104].
New targeted therapies including everolimus (mTOR inhibitor) and sunitinib, along with octreotide have shown improvement in progression-free survival [105–107]. Sunitinib is a multikinase inhibitor that binds to various tyrosine kinase receptors including VEGF receptor. VEGF expression has been associated with risk of metastasis in low-grade neuroendocrine tumors [108]. Anti-VEGF antibody bevacizumab has shown activity in combination with gemcitabine and S1 in PC mouse models [109]. A phase II study by Chan and colleagues looked at 34 patients with gastroenteropancreatic neuroendocrine tumors (44 % pNET, 56 % carcinoid) treated with bevacizumab plus temozolomide. Overall response rate was 15 %, but 33 % of pNET patients (5/15) had a response compared to 0/19 with carcinoid tumors. Both median progression-free (14.3 months vs. 7.3 months) and overall survival (41.7 months vs. 18.8 months) were higher in pNET versus carcinoid [110].
Little research has been pursued in vaccine development for pancreatic neuroendocrine tumors. A case report using autologous DCs pulsed with tumor cell lysate was delivered subcutaneously to a patient with metastatic pNET. A DTH reaction developed and specific T-cell response was noted. The patient had stable disease at 20 months after starting therapy [111].
12.8 Concluding Remarks
Pancreatic cancer continues to be a highly lethal disease in which new therapeutics are desperately needed. Immunotherapy in pancreatic cancer is in its infancy, but gives new targets and new ways to deliver therapeutics to the tumor. Vaccines, mAbs and drug- or radioimmunotherapy has shown promising results in multiple preclinical models and numerous therapies are currently in clinical trials. Although no current immunotherapy is standard of care in pancreatic cancers, they may prove to be key components of treatment in the future.
References
1.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree