The incidence of melanoma has been increasing worldwide. A relationship between melanoma and the immune system was established years ago. Modulating the immune system in the management of different stages of melanoma has been the focus of numerous large randomized trials worldwide. This article reviews the current status of immunotherapy for melanoma, with a focus on the recent promising results from using vaccines, cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibodies, and adoptive cell therapy.
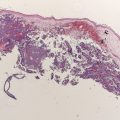
The incidence of melanoma has been steadily increasing worldwide. One in 63 Americans will develop melanoma during his/her lifetime; historically, in 1935, the risk was 1 in 1500. Unlike other solid tumors, melanoma more commonly affects young people. In the 20- to 29-year-old age group, melanoma is the second most common cancer after lymphoma. Local treatment strategies, such as surgery, can achieve local tumor control in the early stages of melanoma; however, the metastatic form of the disease continues to have a dismal prognosis, with a median survival rate of 6 to 9 months.
An early relationship between melanoma and the immune system was established after spontaneous immune-mediated regressions of melanoma were observed. This has inspired many investigators to conduct laboratory and clinical studies to develop an effective and tolerable immunotherapeutic strategy in an attempt to transform it into a highly personalized treatment. An overview of the current status of immunotherapy in patients with melanoma and a focus on some recent success in the field are discussed in this article.
Interferon α-2b
Interferon (IFN) α-2b is a member of a family of cytokines with anticancer, antiviral, and immunomodulatory effects. Interferons were initially discovered as secreted glycoproteins produced by cells in response to a viral infection. Although interferon α-2b is the most widely tested interferon in melanoma, its exact mechanism of action is still unknown. The antitumor actions of IFN may be derived from its activation of signal transducer and activator of transcription (STAT) proteins and also from its direct effects on the host tissues. The clinical manifestations of autoimmunity in patients who received IFN therapy are statistically associated with significant improvements in relapse-free survival and overall survival, which suggests a strong involvement of the immune system in its mechanism of action.
In melanoma therapy, IFN is administered intravenously (IV) or subcutaneously (SC). Its half-life of elimination is approximately 2 hours when given IV and 3 hours when given SC. The most common toxicities of IFN are neutropenia (44%), liver toxicity (29%), and fatigue (24%). During the induction phase, up to 58% of patients might require dose reductions or delays (44% due to toxicity). High-dose IFN (HDI) is the only adjuvant therapy for stages II and III melanoma that has stood the test of time. Its role in this setting was established by the Eastern Cooperative Oncology Group (ECOG) E1684 trial and reassessed in the ECOG E1690, E1694, and E2696 trials. HDI is administered at 20 million units/m 2 /d IV for 4 weeks and 10 million units/m 2 given thrice a week SC for 48 weeks. The relapse and survival status of patients enrolled in the previous 4 studies formed the basis of a pooled analysis of nearly 2000 patients. The objective was to identify prognostic factors as well as relapse rates and survival benefits associated with adjuvant IFN in patients with stage IIB and III melanoma. Relapse-free survival was significantly prolonged (2-sided log-rank P value = 0.006, hazard ratio [HR] = 1.30) with HDI treatment than with observation. Neither the univariate nor multivariate analyses demonstrated a statistically significant survival superiority with HDI treatment compared with observation. This was expected, given that the larger of the 2 trials included in this analysis (E1690) did not show a survival benefit for HDI treatment. Prognostic factors that negatively impacted relapse and survival included ulceration and age greater than 49 years.
A meta-analysis including the 4 aforementioned studies and 8 other European randomized trials using different doses of IFN has been reported. Overall, the analysis showed a statistically significant 17% reduction in the odds of recurrence with IFN (HR = 0.83; 95% confidence interval [CI] 0.77 to 0.90; P = .000003). Similar to the previous analysis, the benefit in overall survival was not significant (0.93, 95% CI 0.85 to 1.02, P = .1). There was evidence of a dose-response relationship, with a trend for the benefit of IFN to increase with increasing doses of IFN.
Based on the available data, it is clear that IFN minimally reduces the risk of relapse after curative-intent surgery. Unfortunately, interferon toxicity is considerable and spans the entire year over which it is given. These toxicities considerably affect quality of life over the course of the treatment. Pegylated IFN (widely used in Europe) and a shorter one-month intravenous schedule of IFN have shown encouraging results but are still not considered to be the standard of care in the United States. One thousand two hundred fifty-six patients with resected stage III melanoma were randomized to observation or pegylated IFN 6 μg/kg/wk for 8 weeks, then 3 μg/kg/wk for an intended duration of 5 years. At 3.8 years median follow-up, 328 recurrence events had occurred in the IFN group compared with 368 in the observation group (HR 0.82, 95% CI 0.71-0.96; P = .01). Patients with an ulcerated primary melanoma seemed to benefit more from pegylated IFN than patients with nonulcerated primaries.
Interleukin-2
Interleukin-2 (IL-2), a natural product secreted by CD4 + T lymphocytes and a T-cell growth factor, was first identified in 1976 but was not produced until 1983, when a biologically active polypeptide characteristic of human IL-2 was formulated. Shortly thereafter, a recombinant form of IL-2 (rIL-2) was shown to have antitumor activity in murine tumor models. The mechanism of this antitumor activity is not well understood. In addition to T lymphocytes, IL-2 stimulates natural killer cells, B lymphocytes, and macrophages, as well as induces lymphokine-activated killer cells in vitro.
Clinical trials conducted using patients with advanced cancer and metastatic melanoma showed antitumor effects associated with high-dose bolus intravenous IL-2 (HDIL-2) administration. Subsequently, HDIL-2 was approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of adults with advanced metastatic melanoma. The efficacy and toxicity profile of HDIL-2 were best described when the data from 270 metastatic melanoma patients treated with HDIL-2 between 1985 and 1993 were analyzed. A bolus intravenous infusion of HDIL-2 (600,000–720,000 IU/kg every 8 hours on days 1–5 and 15–19) was used. An objective response rate of 16% (median response duration, 8.9 months; range, 4 to 106+) with a durable response rate of 4% was reported. Further analysis of the treated patients showed that patients with a good baseline performance status (ECOG of 0), no prior history of systemic therapy, a normal lactate dehydrogenase (LDH) level, and less than 3 organs involved or cutaneous and/or subcutaneous metastases had the highest probability of responding as well as achieving a durable complete response. Autoimmune toxicity, such as treatment-related hypothyroidism, may also be associated with a favorable tumor response. All reported response predictors need to be confirmed in prospective trials.
Although bolus HDIL-2 is more commonly used in the United States, continuous infusion IL-2 schedules remain common treatments in Europe. This commonly has been administered as 18 × 10 6 IU/d for 5 days. Overall response rates have been reported to be comparable to HDIL-2, and survival rates have been documented at 4% at 5 years. Large, randomized controlled trials comparing different IL-2 schedules in melanoma have not been performed.
IL-2 toxicity can involve multiple organ systems, most significantly, the heart, lungs, kidneys, and central nervous system. Most of the toxicity is related to the capillary leak syndrome, which leads to decreased organ perfusion (oliguria and ischemia) due to the accumulation of fluid in the extravascular space. The incidence of toxicities has decreased as clinicians have started to follow strict patient-eligibility criteria and have implemented standardized toxicity prevention guidelines. To minimize the toxicity, IL-2 has been administered in lower doses subcutaneously. This has produced no major responses in melanoma patients.
To enhance its activity in patients with metastatic melanoma, IL-2 has been combined with vaccines. The most studied combination is IL-2 and glycoprotein 100 (gp100):209-217(210M), a peptide vaccine with a higher affinity to HLA-A2 and a better inducer of T-cell stimulation in vitro and in vivo ( Table 1 ). The HDIL-2 and gp100 vaccine is active and has been shown to be more effective in a phase III randomized trial in patients with metastatic melanoma (see Table 1 ). The combination of vaccine and low-dose IL-2 has been proven safe and tolerable but inactive against advanced melanoma.
| References | N | Design | PR | CR | Comments |
|---|---|---|---|---|---|
| 31 | Phase II | 39% | 3% | Objective regression of some brain metastases was seen. | |
| 131 | Phase II | 7% | 9% | 9% progression-free at 30+ months. | |
| 684 | Retrospective | 9% vs 12% (IL-2 alone vs IL2+variety of vaccines) | 4% vs 3% (IL-2 alone vs IL2+variety of vaccines) | Patients who received the gp100:209-217(210M) peptide plus IL-2 showed a strong trend to increased objective responses compared with HDIL-2 alone (22% vs 13%) | |
| 185 | Randomized phase III of the combination vs HDIL-2 alone | 7.5% vs 8% | 2% vs 14% in favor of the combination | Overall response rate and PFS were significantly better with peptide vaccine and HDIL-2 compared with HDIL-2 alone. Trend for greater overall survival in the vaccine arm (18 vs 13 mo) |
Interleukin-2
Interleukin-2 (IL-2), a natural product secreted by CD4 + T lymphocytes and a T-cell growth factor, was first identified in 1976 but was not produced until 1983, when a biologically active polypeptide characteristic of human IL-2 was formulated. Shortly thereafter, a recombinant form of IL-2 (rIL-2) was shown to have antitumor activity in murine tumor models. The mechanism of this antitumor activity is not well understood. In addition to T lymphocytes, IL-2 stimulates natural killer cells, B lymphocytes, and macrophages, as well as induces lymphokine-activated killer cells in vitro.
Clinical trials conducted using patients with advanced cancer and metastatic melanoma showed antitumor effects associated with high-dose bolus intravenous IL-2 (HDIL-2) administration. Subsequently, HDIL-2 was approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of adults with advanced metastatic melanoma. The efficacy and toxicity profile of HDIL-2 were best described when the data from 270 metastatic melanoma patients treated with HDIL-2 between 1985 and 1993 were analyzed. A bolus intravenous infusion of HDIL-2 (600,000–720,000 IU/kg every 8 hours on days 1–5 and 15–19) was used. An objective response rate of 16% (median response duration, 8.9 months; range, 4 to 106+) with a durable response rate of 4% was reported. Further analysis of the treated patients showed that patients with a good baseline performance status (ECOG of 0), no prior history of systemic therapy, a normal lactate dehydrogenase (LDH) level, and less than 3 organs involved or cutaneous and/or subcutaneous metastases had the highest probability of responding as well as achieving a durable complete response. Autoimmune toxicity, such as treatment-related hypothyroidism, may also be associated with a favorable tumor response. All reported response predictors need to be confirmed in prospective trials.
Although bolus HDIL-2 is more commonly used in the United States, continuous infusion IL-2 schedules remain common treatments in Europe. This commonly has been administered as 18 × 10 6 IU/d for 5 days. Overall response rates have been reported to be comparable to HDIL-2, and survival rates have been documented at 4% at 5 years. Large, randomized controlled trials comparing different IL-2 schedules in melanoma have not been performed.
IL-2 toxicity can involve multiple organ systems, most significantly, the heart, lungs, kidneys, and central nervous system. Most of the toxicity is related to the capillary leak syndrome, which leads to decreased organ perfusion (oliguria and ischemia) due to the accumulation of fluid in the extravascular space. The incidence of toxicities has decreased as clinicians have started to follow strict patient-eligibility criteria and have implemented standardized toxicity prevention guidelines. To minimize the toxicity, IL-2 has been administered in lower doses subcutaneously. This has produced no major responses in melanoma patients.
To enhance its activity in patients with metastatic melanoma, IL-2 has been combined with vaccines. The most studied combination is IL-2 and glycoprotein 100 (gp100):209-217(210M), a peptide vaccine with a higher affinity to HLA-A2 and a better inducer of T-cell stimulation in vitro and in vivo ( Table 1 ). The HDIL-2 and gp100 vaccine is active and has been shown to be more effective in a phase III randomized trial in patients with metastatic melanoma (see Table 1 ). The combination of vaccine and low-dose IL-2 has been proven safe and tolerable but inactive against advanced melanoma.
| References | N | Design | PR | CR | Comments |
|---|---|---|---|---|---|
| 31 | Phase II | 39% | 3% | Objective regression of some brain metastases was seen. | |
| 131 | Phase II | 7% | 9% | 9% progression-free at 30+ months. | |
| 684 | Retrospective | 9% vs 12% (IL-2 alone vs IL2+variety of vaccines) | 4% vs 3% (IL-2 alone vs IL2+variety of vaccines) | Patients who received the gp100:209-217(210M) peptide plus IL-2 showed a strong trend to increased objective responses compared with HDIL-2 alone (22% vs 13%) | |
| 185 | Randomized phase III of the combination vs HDIL-2 alone | 7.5% vs 8% | 2% vs 14% in favor of the combination | Overall response rate and PFS were significantly better with peptide vaccine and HDIL-2 compared with HDIL-2 alone. Trend for greater overall survival in the vaccine arm (18 vs 13 mo) |
Biochemotherapy
To improve on the activity of immunotherapy in melanoma, combining different chemotherapy agents with IFN, IL-2, or both has been tested. Several randomized trials compared biochemotherapy to chemotherapy; all concluded that biochemotherapy is associated with increased toxicity. Inconsistent results have been reported on efficacy, with most of the variability coming from single-center studies. The overall response rate was as high as 50% in non-IL-2-based and 48% in IL-2-based biochemotherapy. A doubling in median overall survival has also been reported in small studies (17 vs 8 months).
To further investigate the benefit of biochemotherapy in metastatic melanoma, several meta-analyses have been conducted, with the most recent reported in 2007. In this analysis, 11 randomized trials of chemotherapy ± IFN (1395 patients) and 7 randomized trials of chemotherapy ± IFN and IL-2 (1226 patients) were included. Grade 3 hematologic toxicity was higher with biochemotherapy; the odds ratio for thrombocytopenia was 3.03 (95% CI, 2.16 to 4.25; P = .00001) and for neutropenia/leukopenia was 1.71 (95% CI, 1.25 to 2.34; P = .0008). Biochemotherapy was superior for partial response (odds ratio = 0.66; 95% CI, 0.53 to 0.82; P = .0001) and complete response (CR; odds ratio = 0.50; 95% CI, 0.35 to 0.73; P = .0003). There was, however, no difference between chemotherapy and biochemotherapy in duration of response (42 days), but biochemotherapy did delay the time to disease progression (odds ratio = 0.80; 95% CI, 0.71 to 0.89; P = .0001). There was also no advantage of biochemotherapy in terms of overall survival (OS; odds ratio = 0.99; 95% CI, 0.91 to 1.08; P = .9). Similar to HDIL-2, long-term survival (10 years) has been reported in 6% of patients with metastatic melanoma treated with biochemotherapy. To date, biochemotherapy remains one of the treatments with the highest response rate, which makes it useful in patients with rapidly progressive melanoma by providing symptomatic relief or to render patients with locally and/or regionally advanced disease surgically resectable.
Biotherapy
To improve the response rate of HDIL-2, IFN was tested in combination with high-dose and low-dose IL-2 in melanoma patients but, unfortunately, failed to show significant improvement in the response rate. This combination was recently tested as a maintenance regimen after induction biochemotherapy and was shown to prolong progression-free survival and to improve overall survival compared with other reported multicenter trials of biochemotherapy or chemotherapy. The 12-month and 24-month survival rates were 57% and 23%, respectively. Randomized controlled trials are needed to confirm the results.
Cytotoxic T-lymphocyte antigen-4 blockade
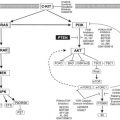
The activation of T cells requires the engagement of CD28 on T cells by B7 molecules expressed on the surface of antigen-presenting cells ( Fig. 1 ). This CD28-B7 interaction induces T-cell proliferation, cytokine secretion, and other effector functions (Cdk-4, Cdk-6, and cyclin D3). T-cell activation leads to the upregulation of cytotoxic T-lymphocyte antigen-4 (CTLA-4) on the T-cell surface, which delivers a negative regulatory signal to T cells after binding to B7. CTLA-4 antibodies inhibit the interaction of B7 and CTLA-4; thus, the inhibitory signal produced by CTLA-4 is blocked, and T-cell activation is prolonged. Early studies in mice showed that an absence of CTLA-4 led to a lymphoproliferative disorder and to lymphocytic infiltration and destruction of major organs.
Two fully human monoclonal CTLA-4 blocking antibodies, tremelimumab (IgG2; Pfizer, New York, NY, USA) and ipilimumab (IgG1; Bristol Myers Squibb, Princeton, NJ, USA), have been evaluated in clinical trials. Although it was suggested that anti-CTLA-4 antibodies deplete the T- regulatory cells, one study evaluating the changes in lymphocyte phenotype and function in melanoma patients receiving anti-CTLA-4 showed that the antitumor and autoimmune effects seen after CTLA-4 antibodies were due to direct activation of CD4 + and CD8 + effector cells, not inhibition or depletion of T regulatory cells.
Both drugs have been tested as a single-agent therapy or in combination with other agents ( Tables 2 and 3 ). Most studies showed an overall response rate of 5% to 15% in patients with metastatic melanoma and reported prolonged survival in some responders. Increased response rate was associated with an increased dose of the CTLA-4 inhibitor and the presence of autoimmune toxicities in some studies. In 2010, a phase III randomized controlled trial showed ipilimumab to improve OS in comparison to gp100 vaccine in previously treated patients with metastatic melanoma. In this study, ipilimumab alone (3 mg/kg) resulted in 1- and 2-year survival rates of 39% and 24%, respectively.
| References | N | Study Design (Phase) | Drug | CTLA-4 Antibody Dose/Schedule | RR | Survival (mo) | Grade 3 & 4 S/E | Comments |
|---|---|---|---|---|---|---|---|---|
| 88 | I/II | Ipi | Up to 10 mg/kg q3W | 5% | 13.5 | 14% | One complete response (21+ mo) | |
| 217 | II | Ipi | 0.3 mg/kg q3W | 0% | 9 | 0% | Significant increase in response with increased dose | |
| 3 mg/kg q3W | 4% | 9 | 7% | |||||
| 10 mg/kg q3W | 11% | 15 | 25% | |||||
| 29 | I | Treme | 0.01−15 mg/kg | 14% | NR | 35% in 10–15 mg/kg | Complete responses (maintained for 34+ and 25+ mo) | |
| 89 | I/II | Treme | 10 mg/kg/mo | 10% | 10 | 27% | Most responses were durable (range, 3–30+ mo) | |
| 15 mg/kg q3Mo | 9% | 11.5 | 13% | |||||
| 155 | II | Ipi | 10 mg/kg q3W | 6% | 10 | 22% | 2-year survival rate 33% | |
| 246 | II | Treme | 15 mg/kg q90D | 7% | 10 | 11% | Durable responses ≥170 d |
| References | N | Study Design (Phase) | Drug | CTLA-4 Antibody Dose/Schedule | RR | Survival (mo) | Grade 3 & 4 S/E | Comments |
|---|---|---|---|---|---|---|---|---|
| 14 | II | Ipi + gp100 | 3 mg/kg IV q3W | 21% | NR | 43% | Accrual ceased after 14 patients because of grade 3 & 4 autoimmune toxicities | |
| 16 | I | Trem + dendritic cell vaccine | 3–15 mg/kg qMo–q3Mo | 25% | NR | 6% | Durable response up to 4 y after study initiation | |
| 36 | I/II | Ipi + HDIL-2 | 0.1–3 mg/kg q3W | 22% | NR | 14% | 75% of responders had a response>11 mo | |
| 56 | II | Ipi+ gp100 | 1–3 mg/kg q3W | 13% | NR | 25% | Autoimmune toxicity associated with response CR ongoing 31 mo | |
| 72 | II | Ipi | 3 mg/kg q4W | 5% | 11 | 8% | No statistical difference in disease control rate in 2 arms. 5 patients remain alive after 5.5+ y | |
| Ipi + dacarbazine (250 mg/m2/d for 5 d) | 3 mg/kg q4W | 14% | 14 | 17% | ||||
| 115 | II | Ipi | 10 mg/kg q3W | 16% | 19 | 47% | Disease control rate was higher in patients with grade 3 & 4 S/E | |
| Ipi + budesonide | 10 mg/kg q3W | 12% | 18 | 55% | ||||
| 139 | II | Ipi + gp100 | 3–9 mg/kg q3W | 17% | 16 | 36% | Immune related adverse events were associated with a greater antitumor response | |
| 676 | III | Gp100 | 1.5% | 6 | 11% | Ipi with or without gp100 vaccine improved overall survival in patients with previously treated metastatic melanoma | ||
| Ipi | 3 mg/kg q3W | 11% | 10 | 23% | ||||
| Ipi+gp100 | 3 mg/kg q3W | 6% | 10 | 17% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree