Lung cancer demography
85 % of lung tumors are NSCLCs
15 % of lung tumors are SCLCs
45 % of NSCLC cases are adenocarcinoma
45 % of NSCLC are squamous cell carcinoma
10 % of NSCLC are large cell
Table 19.2
Displays the key facts about lung tumors
Key facts about lung tumors |
|---|
Peak incidence between 40 and 70 |
Four main types: squamous, small cell anaplastic, adenocarcinoma, and large cell anaplastic |
Clinical division into small cell (SCLC) and non-small cell (NSCLC) |
TNM staging used for NSCLC while either limited or extensive staging for SCLC stages |
Small proportion are operable due to late and advanced presentation |
Tumors can be central or peripheral (more shift lately toward peripheral) |
Overall survival 5–30 % at 5 years depending on histology and stage |
Table 19.3
Describes features of lung tumors that are amenable for surgery
Key features of lung tumors that make them amenable to surgery |
|---|
Tumors <3 cm |
Tumors at least 2 cm distal to the carina |
No contralateral nodal involvement (T3) |
No effusion |
No distant metastasis |
No obstruction of bronchi or pulmonary atelectasis |
No involvement of trachea, great vessels, pericardium, vertebrae, esophagus, diaphragm, or pericardium |
Table 19.4
Comparing the key features of SCLC and NSCLC
SCLC (small cell lung cancer) | NSCLC (non-small cell lung cancer) |
|---|---|
Previously called oat cell | Arise from alveolar cells |
Arise from neuroendocrine cells (Kulchitsky) | Include adenocarcinomas, squamous and large cell tumors |
Secrete peptides | Nonsecretory |
Can cause SIADH (syndrome of inappropriate secretion of ADH) | Adenocarcinomas arise from basal bronchial cells and type II pneumocytes |
19.2 Why Immunotherapy?
Chemotherapy regimens have yielded roughly the same results no matter what the combination albeit the fact that the introduction of tyrosine kinase inhibitors has extended the overall survival of EGFR mutant tumors, as described in Fig. 19.1 [1], hence grew the need for immunotherapy, which started with dendritic cell (DC) therapies. Dendritic cells or antigen-presenting cells (APC) are well known for their plasticity, but the use of dendritic cell immunotherapy has at best yielded only 15 % success rate. This has been due to several factors mainly maturity, timing, antigenic dose, anergy, and several other receptor-ligand interactions. Of particular note has been the recent paradigm shift with the introduction of ipilimumab (Yervoy®) which has boosted the success rates of immunotherapy to well above 20 %, whereby combined with surgery, tissue specific chemotherapy, and immunotherapy, patients have a higher chance of being in remission or possibly cure. Our discussion will henceforth focus on humoral as well as targeted cellular modalities which have been successfully implemented for the last decade.
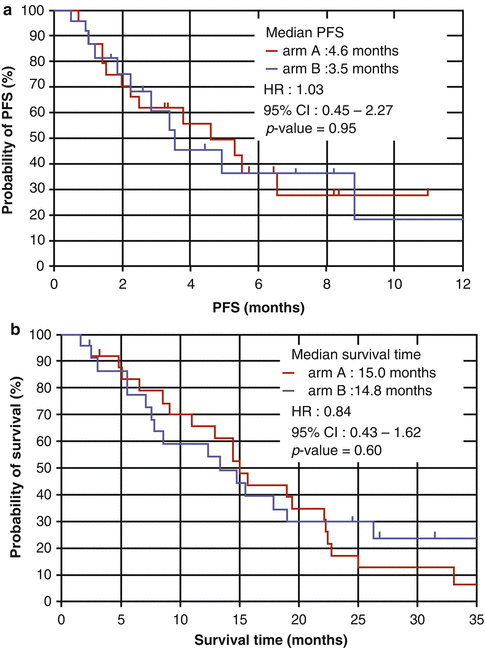

Fig. 19.1
(a, b) Showing a Kaplan-Meier curve showing the probability of progression-free survival (PFS) and overall survival (OS) in patients with carboplatin and paclitaxel (arm a) vs. carboplatin and gemcitabine (arm b). The survival clearly demonstrates that the overall benefits are roughly the same with no statistically significant difference (Image courtesy of BMC Res Notes, Permission Free Open Access Journal [1])
19.3 Antiangiogenic Agents and Monoclonals
19.3.1 Ziv-Aflibercept (Zaltrap®)
Ziv–Aflibercept (Zaltrap ®) is a recombinant fusion protein that binds (scavenges) to VEGF-A, VEGF-B, and placental growth factor and hence does not allow the binding of VEGF to its receptors as shown in Fig. 19.2. This relatively new agent has been randomized in one trial for NSCLC. The VITAL study is a randomized multinational double-blind trial that enrolled 913 patients with non–squamous NSCLC (adenocarcinoma making 80 % of the NSCLC) who have already failed one platinum doublet therapy. The randomization was 1:1 with one subgroup receiving docetaxel (75 mg/m2) and aflibercept (6 mg/kg) every 3 weeks, while the control subgroup received docetaxel only. The primary endpoint was overall survival (OS) and the secondary endpoints were progression-free survival (PFS) and overall response rate (ORR). A total of 12.3 % of the patients had already received prior bevacizumab therapy. The OS in the treatment subgroup was 10.1 months, while it was 10.4 months in the placebo group. PFS and ORR were 5.2 % versus 4.1 % and 23.3 % versus 8.9 % in the treatment and placebo subgroup, respectively [2]. This pioneering study did not show any advantage of scavenger fusion proteins in the treatment of NSCLC.
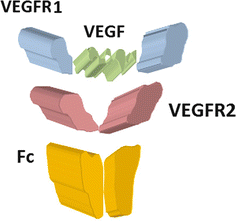

Fig. 19.2
VEGF-Trap/aflibercept is a fully human fusion protein made from a human IgG1 Fc fragment (orange) and domain 3 of VEGFR-2 (Ig) (dark red) as well as an apical VEGFR-1 portion (blue) capable of trapping VEGF (green) and PIGF
19.3.2 Bevacizumab
Bevacizumab or Bev is a humanized monoclonal antibody aimed against VEGF-A, developed by Genentech/Roche, and marketed as Avastin®. VEGF-A is one of the key components in promoting de novo angiogenesis. Bev has been recommended in renal cell carcinoma, carcinoma of the breast (in some countries), glioblastoma multiforme, colonic cancer, and advanced stages of NSCLC. Recent evidence in using Bev has been counterbalanced by the unpredictable nature of the response. This is due to the neutralizing effect of another isoform of VEGF-165b which is an antiangiogenic form of VEGF-A and neutralizes the effect of Bev [3]. This has prompted the development of scavenger agents: ziv-aflibercept and VEGF-receptor inhibitors. So far, none of the VEGFR inhibitors have undergone any clinical trials in relation to pulmonary tumors.
One the first clinical trials employing Bev was E4599 (Eastern Cooperative Oncology Group) which randomized 878 patients with recurrent or advanced non-squamous NSCLC (IIIB or IV) to receive either paclitaxel or carboplatin (N = 444) versus the same therapy plus bevacizumab (N = 434). Chemotherapy was given for 3 weeks for six cycles, while Bev was given every 3 weeks until disease progression or increased toxicity. The primary endpoint was overall survival. The overall difference between the two groups was 2 months in favor of the chemotherapy+Bev group (p = 0.003). Progression-free survival was 6.2 months in the chemotherapy+Bev versus 4.5 months in chemotherapy alone [4]. This trial subsequently led to the European AVAiL trial which confirmed the positive results of adding bevacizumab (7.5 or 15 mg/kg) to gemcitabine and cisplatin in treating non-squamous NSCLC tumors. The results showed a significant benefit in progression-free survival (PFS) (6.7 months in the low-dose group vs. 6.1 for the placebo group and 6.5 months for the high-dose group vs. 6.1 months for the placebo).
Two additional complimentary trials with Bev have emerged as maintenance therapies after chemotherapy. These are AVAPERL and POINTBREAK trials. AVAPERL was a European phase III trial that randomized 362 patients previously treated with pemetrexed, cisplatin, and bevacizumab. The patients were further randomized to receive either maintenance Bev or Bev + pemetrexed every 3 weeks until progression. The trial demonstrated the superiority of maintenance therapy with combined medications (PFS of 10.2 months vs. 6.6 months) but contrasted the futility of having bevacizumab alone in any treatment modality. Finally, POINTBREAK as referred in Fig. 19.3 is a very recent North American trial which was primarily powered for overall survival and PFS as well as RR as secondary endpoints (see below). It proved to be a negative study with absolutely no difference in overall survival between the two arms of the study (13 months) and very similar progression-free survival, but it did show that maintenance therapy was tolerable with significantly higher level of thrombocytopenia in the combined arm.
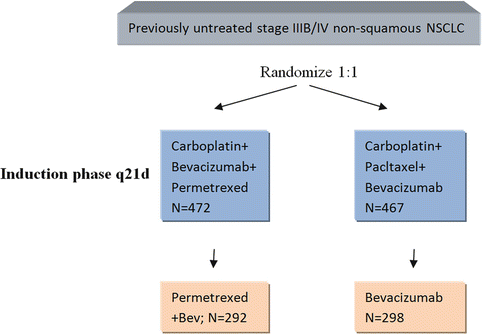

Fig. 19.3
Maintenance phase q21d. Demonstrating the randomization protocol of POINTBREAK trial, both subgroups were given chemotherapy as described in the induction phase for four cycles and then subsequently were maintained on Bev+permetrexed or Bev alone. The maintenance phase was administered every 21 days until disease progression
19.3.3 PD-1 Monoclonal
The birth of the monoclonal antibody against programmed death and its ligand PD1/PD1-L was heralded as a great leap in immunotherapy. PD-1 is a member of the CD28 family. It is expressed on T cells, memory cells, and regulatory T cells. The binding of PD-1 to its corresponding ligand PD-1L downregulates the activity of the T cell and inhibits it. The expression of PD-1L heralds poor tumor prognosis. PD1/PD-1L is regarded as a secondary signal after the initial interaction of T-cell receptor with antigen and MHC—Fig. 19.4. This secondary signaling pathway acts as an inhibitor of death. Henceforth, an antibody blocking the PD1 motif will cause cell death. BMS-936558 is an IgG4 monoclonal that blocks the docking of PD-1L and PD-2L onto PD-1. The current literature demonstrates good safety and efficacy in a preliminary phase I study by Brahmer and colleagues, whereby 39 patients with advanced solid tumors were given dose-escalating infusions of 0.3, 1, 3, and 10 mg/kg. The patient pool contained melanoma, renal cell carcinoma (RCC), colorectal carcinoma, NSCLC, and castration-resistant prostatic carcinoma (CRPC). The treatment was well tolerated; however, in nine patients observed, the success of the treatment was correlated with PD-1L expression [5]. Yet, in another study, SL Topalian and colleagues recruited 296 patients with advanced solid tumors who were given anti-PD-1 antibody at doses from 0.1 mg/kg up to 10 mg/kg every 2 weeks. Response was assessed after 8 cycles, but patients were given up to 12 cycles of therapy until complete response or disease progression. Of the 236 assessed, objective responses were more common in patients with NSCLC, melanoma, and RCC; this was further reflected with the response rate of PD-1L-expressing tumors which was 36 % (9 out of 25) and totally negative in the tumors not expressing PD-1L (Fig. 19.5). Overall response rate in the NSCLC subgroup was 18 % (14 out of 76), 28 % in the melanoma subgroup, and 27 % in the RCC subgroup [6]. Although this was not a randomized trial, it did prove the principle that in tumors expressing PD-1L, it is well worth examining the option of using anti-PD1 antibodies. Another phase II multicenter trial (CA 209-063) with BMS 936558 will be looking into patients with advanced squamous cell NSCLC who have received at least two prior chemotherapy regimens. The study is still recruiting patients and should be finished by 2014. Thus, although we do not have a large well-powered randomized trial with BMS 936558, this relatively new monoclonal antibody could hold much promise in the future.
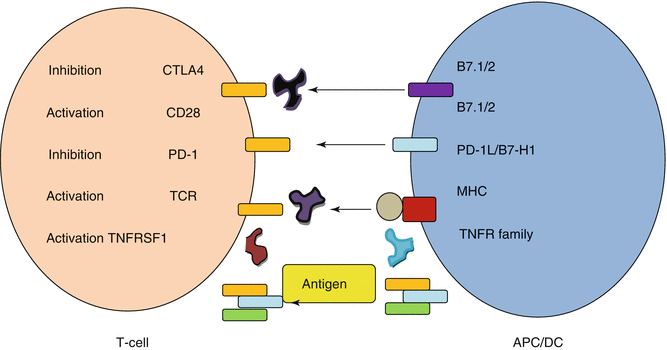
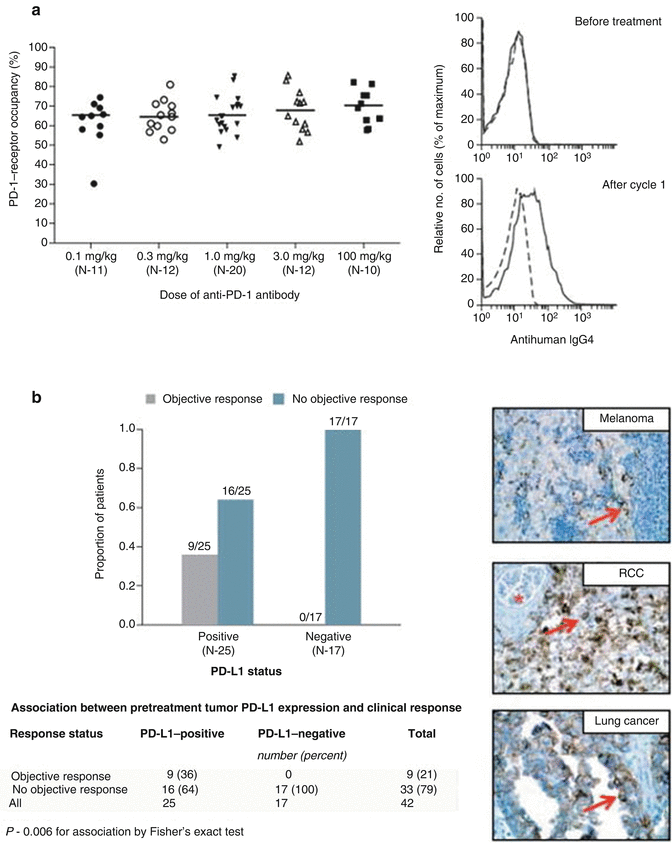

Fig. 19.4
Demonstrates the interaction of T cell with antigen-presenting cell. The antigen (yellow) is sandwiched between the MHC and the T-cell receptor. Secondary activating signals between CD28 and B7.1 are important in consolidating an immune response. Inhibitory responses mainly between PD-1 and PD-1L as well as CTLA4 and B7.1 are important in the abrogation of the immune response. In this representation, BMS936558 (purple) and ipilimumab (black) inhibit these inhibitory interactions and thus cause an inhibition of inhibitory stimuli, therefore priming the T cell

Fig. 19.5
(a) Shows PD-1-receptor occupancy by anti-PD-1 antibody. The graph at the left shows PD-1-receptor occupancy on circulating T cells in 65 patients with melanoma after one cycle (8 weeks) of treatment at a dose of 0.1–10.0 mg per kilogram every 2 weeks. Bars indicate median values. The graphs at the right show the flow cytometric analysis of PD-1-receptor occupancy on CD3-gated peripheral blood mononuclear cells from a patient with melanoma who received 0.1 mg per kilogram, before treatment (top) and after one treatment cycle (bottom). Dashed lines indicate isotype staining controls, and solid lines antihuman IgG4. Panel (b) shows the correlation of pretreatment tumor cell-surface expression of PD-1 ligand (PD-L1), as determined with immunohistochemical analysis of formalin-fixed, paraffin-embedded specimens, with an objective response to PD-1 blockade in 42 patients with advanced cancers: 18 with melanoma, 10 with non-small cell lung cancer, 7 with colorectal cancer, 5 with renal cell cancer, and 2 with castration-resistant prostate cancer. Tumor cell-surface expression of PD-L1 was significantly correlated with an objective clinical response (graph at the left). No patients with PD-L1-negative tumors had an objective response. Of the 25 patients with PD-L1-positive tumors, two who were categorized as not having had a response at the time of data analysis are still under evaluation. Shown at the right are immunohistochemical analysis with the anti-PD-L1 monoclonal antibody 5H1 in a specimen of a lymph node metastasis from a patient with melanoma (top), a nephrectomy specimen from a patient with renal cell cancer (RCC) (middle), and a specimen of a brain metastasis from a patient with lung adenocarcinoma (bottom). The arrow in each specimen indicates one of many tumor cells with surface membrane staining for PD-L1. The asterisk indicates a normal glomerulus in the nephrectomy specimen, which was negative for PD-L1 staining (Images and data reproduced from Topalian et al. [6], with permission from the Massachusetts Medical Society)
19.3.4 Cetuximab
Cetuximab is a monoclonal antibody that targets epidermal growth factor receptor (EGFR), which is an extracellular receptor with complex downstream signaling. Cetuximab is an IgG1 chimeric antibody that prevents the dimerization of its receptor with five- to tenfold affinity compared to its native ligand, thus preventing further receptor action. It also mediates antibody-dependent cell-mediated cytotoxicity (ADCC) and downregulation of the extracellular receptor. The dogma of using cetuximab in NSCLC is based upon the fact that 80 % of the diagnosed patients have high expression of EGFR extracellularly on immunohistochemical analysis. However, further analysis in colorectal cancer (CRC) and squamous cell carcinoma of the head and neck (SCCHN), whereby cetuximab and another antibody, panitumumab, are used to treat advanced stage disease, revealed that mutational variations in K-ras, a further downstream GTPase, have a significantly different outcome. Colorectal cancer and squamous cell carcinoma of the head and neck patients treated with EGFR monoclonal antibodies against wild-type K-ras have a significantly better outcome than those harboring mutations as demonstrated from the phase III CRYSTAL and EXTREME trials, respectively. In diametric contrast, analysis of K-ras mutations in NSCLC, whether wild type or mutated, this correlation has not been demonstrated, thus suggesting that further biomarkers are required in the determination of prognosis. Crucial to the use of cetuximab in NSCLC has been the FLEX trial which has shown that the addition of cetuximab to first-line chemotherapy in advanced NSCLC significantly improved survival in patients as compared to chemotherapy alone. In 1,121 patients with positive immunohistochemistry for EGFR staining that were further subdivided into high (345) and low (776), the overall survival in the high EGFR-expressing chemotherapy plus cetuximab group versus the chemotherapy alone group was significantly higher (12.0 months vs. 9.6 months), whereas the low EGFR-expressing subgroup showed no overall benefit (9.8 cetux +chemo. vs. 10.3 months for chemotherapy alone) [7]. However, there has been some reluctance in using cetuximab in some communities due to high cost and availability of oral tyrosine kinase inhibitors that act further downstream from EGFR receptor.
19.3.4.1 The EGFR Inhibitor Rash
A well-known dermatitic rash develops with use of EGFR inhibitors as demonstrated in Fig. 19.6. The rash develops in almost two thirds of patients using EGFR inhibitors. The association of the rash with anticancer activity was observed by Saltz and colleagues with increasingly apparent association between cutaneous toxicity and favorable EGFR activity. Almost all clinical trials report a positive association; nonetheless, there is a significant reporter bias. The underlying mechanism of action is difficult to interpret as clinicians have aimed to relate tumor EGFR with skin EGFR. However, unlike other tumor predictors of EGFR (mutant/amplification), the same cannot be said about the skin. Additionally, the tumor mutations are somatic mutations and do not involve the skin. Alternatively, a much pragmatic explanation would be that the rash is a clear indication of adequate drug exposure whether it is EGFR inhibitors or tyrosine kinase inhibitor (TKI) like gefitinib. To simplify matters, two mechanisms have been postulated: one is the inhibition of the skin itself and the second one is the result of systemic immunologic reaction. The last finding is based upon the fact that in some patients, the rash is self-limiting in a small number of patients and improves over time while being managed with steroids. While the inhibitor-associated rash is clinically related to anticancer activity, the treatment of this rash will permit more patients to benefit from inhibitor treatment [8].
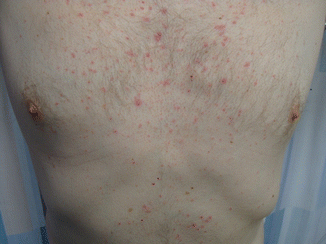
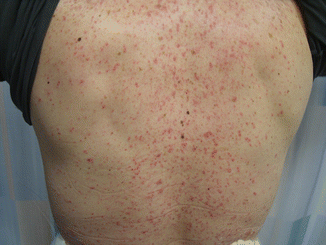

Fig. 19.6
Showing a patient receiving with rash after receiving four cycles of cetuximab (The permission was granted by the patient. Copyright of the chapter author)

19.3.4.2 The Measurement of Immune Response in Monoclonals
Before undertaking the discussion of the next monoclonal antibody, I would like to take the opportunity to explain the major differences between standardized response rates as classified by the World Health Organization and the immune-related criteria (IRC). Traditional dogma dictates that the response to chemotherapy should be evident in a few weeks. However, immunotherapy does not obey those rules; it requires the activation of T cells and their proliferation, but for this to achieve clinically measurable antitumor effect, it takes a few months, and finally, the ultimate outcome on survival is possibly looking at a few years.
Wolchok and colleagues elegantly described the patterns of response in ipilimumab which is clearly shown in Fig. 19.7 and Table 19.5. This describes the patterns of response and warrants clinicians and immunologists that albeit the fact that tumors might eventually decrease in overall size, there can be an initial increase; this is termed pseudoprogression which might be evident for up to 6 months in some cases [9]. It is important to appreciate the clinical significance of pseudoprogression as modern-day biologicals are likely to have a delayed onset of action and possibly a longer duration of action. Additionally, these new agents might prompt clinicians to terminate therapy early, although it might be that the immune response is just starting to recruit the necessary reactive cells.
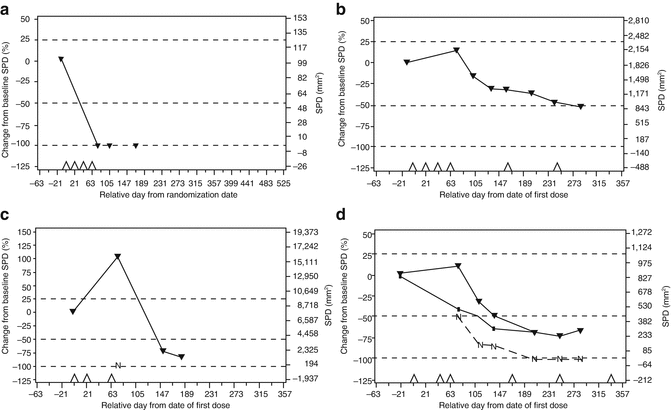

Fig. 19.7
Showing the patterns or response to ipilimumab which were observed in advanced melanoma. Four patterns of response have been observed in advanced melanoma patients treated with ipilimumab at 10 mg/kg in the CA184-008 and CA184-022 trials. (a) Immediate response; (b) “stable disease” with slow, steady decline in total tumor volume; (c) response after initial progression; (d) initial mixed response. SPD sum of the product of perpendicular diameters, N tumor burden of new lesions (c, d). Panel (d) top line, total tumor burden; middle line, tumor burden of baseline lesions; bottom line, tumor burden of new lesions. Triangles on the x-axis: ipilimumab dosing time points; dashed lines, thresholds for response or progressive disease/immune-related progressive disease [9] (Reprinted by permission from the American Association for Cancer Research: Wolchok et al. [9])
Table 19.5
Displaying the differences between WHO and immune-related response criteria (irRC)
CR | PR | SD | PD | |
WHO criteria | All lesions gone | SPD of index lesions decreases ≥50 % | SPD of index lesion of neither CR, PR, nor PD | SPD of index lesion increases by 25 % and/or new lesions develop |
New lesions not allowed | ||||
irCR | irPR
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||
