Agent
n
Phase
References
Proleukin (IL-2)
255
Phase II
Fyfe G. et al. [19]
IFN-α
350
Meta-analysis
Medical Research
(vs medroxyprogesterone acetate)
Council Renal Cancer Collaborators [22]
IFN-α+ VLB
160
RCT
Pyrhonen S et al.[23]
(vs VLB alone)
IFN-γ
197
RCT
Gleave et al. [24]
Placebo
Table 12.2
Therapeutic RCC vaccines
Agent | n | Phase | References |
|---|---|---|---|
IMA901 | 28 | Phase I | Walter S et al. [27] |
68 | Phase II | ||
Ongoing | Phase III | NCT01265901 | |
Nephrectomy + Reniale® | 379 | Phase III | Jocham D. et al. [28] |
Nephrectomy | |||
MVA-5 T4 | 733 | Phase III | Amato RJ. et al. [30] |
Placebo | |||
Vitespen (formerly Oncophage®) | 818 | Phase III | Wood C. et al.[31] |
TG-4010 | 37 | Phase II | Oubard S. et al.[32] |
AGS-003 | 21 | Phase II | Amin A. et al. [34] |
AGS-003 | Ongoing | Phase III | NCT01826877 |
DC-vaccine | 148 | Meta-analysis | Draube A. et al. [35] |
DC-CIK | Ongoing | Phase III | NCT00862303 |
12.2 Cytokine Therapy (IFN-α, IFN-γ, and IL-2) for Metastatic RCC
For metastatic RCC, cytokine therapies with a focus on interferon (IFN-α) and interleukin (IL-2) have been studied (Table 12.1). However, based on elucidation of the mechanisms underlying RCC onset and progression, many molecular targeted drugs have been developed. Currently, molecular targeted drugs are used as the first-line treatment for metastatic RCC.
Bolus high-dose intravenous recombinant human IL-2 treatment, particularly in patients with metastatic RCC, was reported in 1985 and 1987 [17, 18]. Based on the results obtained, cytokine therapy for oncological use was developed. For IL-2, no randomized controlled trial (RCT) has yet been performed, but the response rate in the phase II trial was 14 % and the CR rate 5 % [19]. Patients who obtained CR showed long-term survival, raising the possibility of a potentially curative treatment. As a result of this clinical trial, IL-2 was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic RCC in the 1990s. In addition, the objective response rate (ORR) to cytokines was reportedly 12.9 % and the CR rate was 3.6 % in a meta-analysis [20]. Furthermore, IL-2 therapy was found to be effective, achieving long-term clinical responses [21].
There are also two reports of homogeneous RCT examining IFN-α for metastatic RCC [22, 23]. One was from the Medical Research Council Renal Cancer Collaborators [22]. Their 350 cases in the target population were divided into two groups, one treated with IFN-α, the other with medroxyprogesterone. The proximity effect, as indicated by nonprogressive survival, showed IFN-α to be effective, and the OS findings were consistent with this observation. Another RCT was reported by Pyrhonen [23]. The 160 cases in the target population were divided into two groups, one receiving a combination of vinblastine (VBL) and IFN-α and the other VBL only. The combination of IFN-α and VBL was more effective than VBL alone in terms of the proximity effect, nonprogressive survival, and OS. Based on these results, the efficacy of IFN-α monotherapy for advanced RCC has been confirmed.
On the other hand, with respect to IFN-γ therapy for metastatic RCC, Gleave reported different RCT findings [24]. The target population of 197 patients was divided into two groups, one given IFN-γ and the other a placebo. However, IFN-γ had no impact on either the proximity effect or nonprogressive survival.
Currently, IFN-α therapy appears to be inferior, in terms of both the response rate and nonprogressive survival, as compared to some of the molecular targeted drugs [7, 8]. Therefore, molecular targeted therapy has recently been standardized. Once the responsiveness of patients to IFN-α and IL-2 therapy has been determined, it will become necessary to review the future role of cytokine monotherapy regimens for RCC.
12.3 Therapeutic RCC Vaccines
It has been suggested that cancer vaccines are safe as well as showing efficacy against some carcinomas [25, 26]. IMA901, Reniale®, TroVax® (MVA-5 T4), Vitespen (formerly Oncophage®), TG4010, AGS-003, and the dendritic cells (DC) vaccines are now recognized as therapeutic RCC vaccines (Table 12.2).
IMA901
IMA901 is composed of multiple tumor-associated peptides (TUMAPs). In a phase I study [27], the IMA901vaccine induced T-cell immune responses. In addition, the regulatory T cell number needed to suppress the immune response was low. Moreover, in a randomized phase II trial, a single dose of cyclophosphamide prior to IMA901 vaccine administration reduced the number of regulatory T cells (Treg) and confirmed that immune responses to multiple TUMAPs were associated with longer OS. Currently, a randomized phase III trial is being conducted to determine the clinical benefits of treatment with IMA901. Three hundred and forty patients were randomized to a sunitinib alone regimen or a combination of first-line sunitinib and IMA901.
Reniale® (Autologous RCC-Tumor Lysate Cell-Based Vaccine)
Reniale® is an autologous RCC-tumor lysate cell-based vaccine. In a phase III trial, 379 metastatic RCC patients who had undergone nephrectomy in an adjuvant setting were given Reniale® [28]. PFS rates at 5 years and 70 months were 77·4 % and 72 % in the vaccine group and 67·8 % and 59·3 % in the control group, respectively. Adjuvant treatment with Reniale® after radical nephrectomy thus appeared to be beneficial.
TroVax® (MVA-5 T4)
TroVax® (MVA-5 T4) is a therapeutic vaccine targeting the tumor antigen 5 T4 expressed in human cancer cells [29]. In a randomized, placebo-controlled phase III trial, MVA-5 T4/placebo in combination with either sunitinib, IL-2, or IFN-α as first-line metastatic RCC therapy produced no significant increase in OS [30]. However, patients treated with MVA-5 T4 plus IL-2 with a good prognosis showed a significantly better OS than comparable patients receiving the placebo plus IL-2 (HR, 0.54; 95 % CI, 0.30–0.98; P = 0.046). These findings indicated that there might be subsets of patients who would benefit from MVA-5 T4.
Vitespen (Formerly Oncophage®)
Vitespen (formerly Oncophage®) is composed of an autologous tumor-derived heat shock protein (glycoprotein 96) peptide complex (HSPPC-96) [31]. In a phase III trial, there was no difference in recurrence-free survival between the vitespen group and those who received no treatment after nephrectomy for RCC.
TG4010
TG4010 is a therapeutic vaccine targeting the vaccinia virus expressing MUC1, which is overexpressed in RCC and is related to RCC progression [32]. No objective clinical response (clinical response, safety, time to treatment failure, OS, and immune response) was observed in a phase II study evaluating TG4010 efficacy and tolerability, alone or in combination with IFN-α2a and IL-2, for metastatic RCC. Stable disease for more than 6 months was reported in 5 of 27 evaluable patients (18 %) receiving TG4010 alone and 6 of 20 patients (30 %) given TG4010 plus cytokines. MUC1-specific CD8+ T-cell responses were associated with OS [33].
AGS-003
AGS-003 is an autologous immunotherapy employing a DC-based vaccine in which mature DCs are electroporated with amplified tumor RNA plus synthetic CD40-ligand (CD40L) RNA. In a phase II study, AGS-003 was evaluated in combination with sunitinib in intermediate and poor-risk, treatment-naïve patients with metastatic RCC eligible for nephrectomy [34]. Median PFS was 11.2 months (95 % CI: 6.0, 19.4) and median OS was 30.2 months (95 % CI: 9.4, 57.1) for all patients. AGS-003 showed no major toxicity other than grade 1 local reactions. In addition, the magnitude of the increase in the absolute number of cytotoxic (CD8 (+), CD28 (+), CD45RA (−) effector/memory) T cells (CTLs) correlated with OS. Currently, a phase III trial is underway to examine the combination of AGS-003 plus sunitinib versus sunitinib alone, in newly diagnosed unfavorable-risk metastatic RCC patients.
DC Vaccines
Dendritic cells (DC) are potent antigen-presenting cells that play a role in the induction of antigen-specific T-cell responses. In a meta-analysis of 12 trials, objective response rates averaged 12.7 % and clinical benefit was seen of 48 % of RCC cases [35]. However, DC subtypes and antigen types differed among these trials.
12.4 Immune Checkpoint Blockade Therapy for RCC
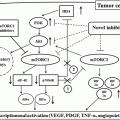
Recent clinical advances in cancer immunotherapy have been remarkable. This growing class of therapeutic agents is designed to modulate and activate a patient’s physiological immune activities to fight cancer and includes a wide variety of approaches [36]. “Immune checkpoint blockade” is the leading strategy and has been actively investigated in clinical trials during this decade [37–39]. It is based on blocking monoclonal antibodies (Abs), “immune checkpoint inhibitors,” which antagonize the “immune checkpoint molecules” expressed on immune cells, or their ligands on immune cells and/or certain types of tumor cells. The immune checkpoint molecules, by interacting with their ligands, send inhibitory intracellular signals to suppress immune cell activities. This inhibitory axis ordinarily attenuates excessive immune responses and follows normal tissue injury; in other words it maintains self-tolerance [40, 41]. The knockout mouse model with impairment of this pathway develops an autoimmune-like syndrome, which is potentially lethal, and prominent T-cell infiltrations into multiple organs. One of the mechanisms by which cancer cells evade antitumor immune attack is hijacking this immunosuppressive pathway, by either directly utilizing the immune checkpoint molecules or indirectly recruiting regulatory immune cell subsets [42]. Based on our understanding of these mechanisms, immune checkpoint blockade, which is often compared to “releasing the brakes on immune cells,” aims to redirect and boost antitumor immune activities. Due to this mechanism of action, unlike anti-angiogenic therapy or other antitumor Abs such as anti-CD20 rituximab and anti-HER2 trastuzumab, target molecules on the tumor cell surface do not need to be identified (Fig. 12.1 ). Based on the encouraging data obtained from animal models, a number of early clinical trials for patients with refractory advanced malignancies have been launched.
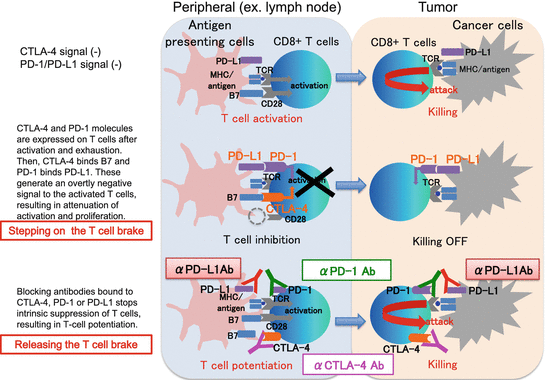

Fig. 12.1
Mechanisms of action of immune checkpoint inhibitors
Several agents have been rapidly advanced into phase III trials, and some have even obtained regulatory approval based on their safety profiles and durable antitumor efficacies. Ipilimumab (BMS, Princeton, NJ), which is directed against the CTL antigen-4 (CTLA-4), is a first-in-class drug that was approved for the treatment of advanced melanoma by the US FDA in 2011 [43, 44]. The long-term safety and efficacy profiles of ipilimumab for melanoma provide a platform for the clinical development of this class of agents targeting several other immune checkpoint molecules [45, 46]. At present, intensive investigations of these agents, as monotherapy or with combination therapy, in a variety of tumors are underway [47–49]. In the following sections, we will focus on the clinical development of immune checkpoint inhibitors for RCC patients (summarized in Table 12.3).
Table 12.3
Immune checkpoint blockade as a monotherapy for RCC
Target | Agent | Patient setting | Development status | Trial ID | Phase | Trial status | N (N planned, if recruiting) | Regimen | Primary end point | Response | Survival | AEs | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTLA-4 | Ipilimumab | ≧ 2nd line | NCT00057889 | II | Completed | 61 | (3 mg/kg every 3 weeks) vs. (3 mg/kg 1d followed by 1 mg/kg every 3 weeks) | Safety | ORR (in 40 pts. receiving 3 mg/kg): 12.5 % | NA | Gr. 3–4 irAEs: 33 % | [54] | |
PD-1 | Nivolumab | ≧ 2nd line | Approved | NCT00441337 (MDX1106–01) | I | Completed | 1 | 3 mg/kg, three doses | Safety | PR after three doses CR after 4 years from the last dose | NA | Hypothyroidism | |
NCT00730639 (MDX1106–03) | Ib | Ongoing (not recruiting) | 34 | 1 vs. 3 mg/kg, every 2 weeks | Safety | ORR: 29 %, median duration of 12.9 mo | Median PFS: 7.3 mo median OS: 22.4 mo | Gr. 3–4 trAEs: 18 % | |||||
NCT01358721 | I | Ongoing (not recruiting) | 80 | 0.3 vs. 2 vs. 10 mg/kg, every 3 weeks | Immunomodulatory activity | NA | NA | NA | https://www.clinicaltrials.gov | ||||
NCT01354431 | II | Ongoing (not recruiting) | 168 | 0.3 vs. 2.0 vs. 10.0 mg/kg, every 3 weeks | PFS | ORR (0.3 vs. 2.0 vs. 10.0): 20 % vs. 22 % vs. 20 % | Median PFS: 2.7 vs. 4.0 vs. 4.2 mo median OS: 18.2 vs. 25.5 vs. 24.7 mo | Gr. 3–4 trAEs: 11 % | [68] | ||||
NCT01668784 (CheckMate-025) | III | Ongoing (not recruiting) | 820 | (Nivo: 3 mg/kg every 2 weeks) vs. (eve: 10 mg daily) | OS | ORR (nivo vs. eve): 25 % vs. 5 % | Median PFS: 4.6 vs. 4.4 mo median OS: 25.0 vs. 19.6 mo | Gr. 3–4 irAEs: 19 % vs. 37 % | [69] | ||||
Pembrolizumab | As neoadjuvant | NCT02212730 | I | Ongoing (recruiting) | 36 | (pemb + surgical resection) vs. (surgical resection only) Pemb: 200 mg, every 3 weeks, up to three cycles | Safety | NA | NA | NA | |||
PD-L1 | Atezolizumab (MPDL3280A) | ≧ 2nd line | NCT01375842 | I | Ongoing (recruiting) | 604 | 3 vs. 10 vs. 15 vs. 20 mg/kg, every 3 weeks | Safety | ORR: 15 % | Median PFS: 5.6 mo median OS: 28.9 mo | Gr. 3 trAEs: 17 %, Gr. 3 irAEs: 4 % No Gr. 4–5 AE occurred | [75] | |
BMS-936559 | ≧ 2nd line | NCT00729664 (MDX1105–01) | I | Completed | 17 | 10.0 mg/kg, every 2 weeks | Safety | ORR: 12 %, SD (≧24 weeks): 41 % | PFS (@24 weeks): 53 % | NA | [76] |
12.4.1 Anti-CTLA-4 Ab
CTLA-4 (also known as CD152) is a member of the CD28 family of co-inhibitory receptors expressed on the surfaces of Foxp3+ Treg cells and activated by T cells [40, 50]. CTLA-4 binds its ligands, B7.1 (CD80) and B7.2 (CD86), expressed on antigen-presenting cells. The inhibitory effect on T-cell activities is derived mainly from two mechanisms. One is the competition between CTLA-4 and costimulatory receptor CD28, for binding shared ligands (B7.1 and B7.2). As CTLA-4 has stronger binding affinity than CD28, it reduces CD28-derived T-cell stimulation. The other is a direct intracellular signal that CTLA-4 sends to attenuate the T-cell receptor (TCR)-mediated signaling pathway [51]. It has also been suggested that the anti-CTLA-4 Ab ipilimumab, which has a humanized IgG1-type Fc domain with the highest affinity for Fcγ receptors among the known human IgG isotypes, might have an exceptional mechanism of action [52]. In a mouse model, it was shown that intra-tumoral Tregs with higher CTLA-4 expression relative to other T-cell subsets were preferentially depleted by antibody-dependent cellular cytotoxicity (ADCC) [53]. On the basis of encouraging preclinical data demonstrating this antitumor effect, CTLA-4-blocking antibodies have been extensively evaluated in melanoma and other histological tumor types.
Ipilimumab is a fully humanized IgG1 monoclonal Ab that blocks CTLA-4. A phase II trial compared two cohorts of RCC patients receiving either continuous 3 mg/kg or one dose of 3 mg/kg followed by 1 mg/kg of ipilimumab (both every 3 weeks) [54]. In the former (higher-dosing) cohort, treated with the approved and commonly used regimen, 5 of 40 patients including IL-2 refractory cases showed a partial response (PR), three of whom experienced a durable (> 1 year) response. Grade 3–4 immune-related adverse events (irAEs) were seen in 33 % of all patients.
12.4.2 Anti-PD-1 and Anti-PD-L1 Ab
Programmed cell death protein-1 (PD-1, also known as CD279) is a member of the CD28 family of co-inhibitory receptors, which are inducibly expressed on the T, B, and NK cells [40, 55]. The ligands of PD-1 are PD-L1 (B7-H1) and PD-L2 (B7-H2), expressed on antigen-presenting cells [56]. The difference between PD-1 and CTLA-4 is that CTLA-4 functions in the priming phase of T-cell activation, PD-1 in the effector phase. PD-1 has a cytoplasmic domain that sends inhibitory signals to the TCR-derived signaling pathway [51]. It has been shown that PD-L1 is expressed in some tumor types including RCC [57–59] to evade antitumor immune surveillance and eradication [60]. PD-1 and/or PD-L1 expression in tumor tissues from RCC cases is associated with tumor aggressiveness and a poor prognosis [61, 62]. Preclinical and clinical studies have shown that blocking the PD-1-PD-L1/2 pathway leads to reactivating antitumor immune responses and tumor regression [63].
Nivolumab is a fully human IgG4 monoclonal antibody against PD-1 [63]. A first-in-human, dose-escalating phase I clinical trial evaluated nivolumab in 39 patients with advanced metastatic solid tumors, including one RCC patient who had VEGF inhibitor refractory disease [64]. Nivolumab was generally well tolerated, and the maximum tolerated dose with a single administration was not defined. The RCC patient showed a durable PR after three doses of 10 mg/kg of nivolumab and then a CR after 3 years without any antitumor therapy [65]. A subsequent phase I trial evaluated nivolumab in a larger cohort of patients, including 34 RCC patients with advanced disease [66]. The long-term follow-up data from the RCC cohort was recently published [67]. The patients, 71 % of whom had already been treated with 2–5 regimens, received either 1 mg/kg (N=18) or 10 mg/kg (N=16) of nivolumab every 2 weeks in an 8 week cycle up to 96 weeks. In all patients, the ORR was 29 %, with a median duration of 12.9 months. The median PFS was 7.3 months (95 % CI: 3.6–10.9), and the median OS was 22.4 months (95 % CI: 12.5, not estimable). Grade 3–4 adverse events (AEs) occurred in approximately 18 % of patients. Based on the encouraging results of these early studies, a randomized, dose-ranging phase II trial was conducted [68]. The 168 RCC patients received either 0.3 (N = 60), 2.0 (N = 54), or 10.0 (N = 54) mg/kg of nivolumab administered once every 3 weeks. There were 118 patients (70 %) who had received at least two prior systemic therapies. The primary end point was PFS. The ORRs were 20 %, 22 %, and 20 % in the 0.3-, 2.0-, and 10.0-mg/kg cohorts, respectively. There was no dose-response relationship for PFS: the median PFS rates were 2.7 (80 % CI: 1.9–3.0), 4.0 (80 % CI: 2.8–4.2), and 4.2 (80 % CI: 2.8–5.5) months, respectively (P = 0.9). The median OS rates were 18.2 (80 % CI: 16.2–24.0), 25.5 (80 % CI: 19.8–28.8), and 24.7 (80 % CI: 15.3–26.0) months, respectively. Compared with the previous phase I trial, this study showed lower ORR and PFS but similar OS. A phase III RCT (CheckMate 025 trial) compared nivolumab with the mTOR inhibitor everolimus, which is a standard salvage therapy, in previously treated RCC patients [69]. In July 2015, this study was stopped ahead of schedule after the independent data monitoring committee found significant survival superiority in those receiving nivolumab as compared to everolimus. In total, 820 patients had been randomly assigned at a 1:1 ratio to nivolumab (3 mg/kg every 2 weeks; N = 410) or everolimus (10 mg daily; N = 411). The primary end point was OS. The ORR was 25 % in the nivolumab vs. 5 % in the everolimus cohort (odds ratio 5.98; 95 % CI: 3.68–9.72; P < 0.001). The median PFS was 4.6 (95%CI: 3.7–5.4) vs. 4.4 (95%CI: 3.7–5.5) months (HR: 0.88; 95 % CI: 0.75–1.03; P = 0.11), and the median OS was 25.0 (95%CI: 21.8-NE) vs. 19.6 months (95%CI: 17.6–23.1) (HR 0.73; 98.5 % CI: 0.57–0.93; P = 0.002), respectively. Grade 3–4 irAEs occurred in 19 % of the patients receiving nivolumab, an incidence significantly lower than that in the everolimus cohort (37 %).
The Checkmate 025 trial validated earlier trials showing OS prolongation with nivolumab as salvage therapy. The lack of consistent PFS extension might be attributable to variable patterns of responses to immune checkpoint blockade, with some of the treated patients initially showing progression on computed tomographic scans due to transient inflammation giving the appearance of tumor enlargement. This “pseudo-progression” highlights the necessity of establishing specific response criteria for cancer immunotherapy [70, 71]. Another clinically important point underscored by this study was the lack of an association between PD-L1 expression in the tumor and the response to nivolumab. Pretreatment biomarkers predicting therapy responsiveness and/or toxicity are urgently needed to select patients who would actually benefit from this class of therapy. As to this point, which we will discuss separately (see section below), nivolumab was approved by the US FDA for patients with advanced RCC refractory to anti-angiogenic therapy, in November 2015.
Atezolizumab (MPDL3280A) is a humanized IgG-1κ monoclonal Ab to PD-L1 [72]. It has a genetically modified Fc domain that is involved in impairing the ADCC-dependent cellular cytotoxicity directed to PD-L1-expressing cells. Following studies in other types of tumors [73, 74], atezolizumab was recently evaluated in a phase I dose-escalation study in 70 patients with previously treated advanced RCC [75]. The doses ranged from 3 to 20 mg/kg (administered every 3 weeks). Atezolizumab was well tolerated and no maximum tolerated dose was defined. Grade 3 treatment-related AEs and irAEs were seen in 17 % and 4 % of patients, respectively, but no grade 4–5 AEs occurred. The ORR was 15 % in 62 evaluable patients. There was a trend toward a higher response rate in patients with PD-L1 expression in TILs. Importantly, an antitumor response was seen in patients with known poor prognostic features, including high Fuhrman grade 4 and/or sarcomatoid features (22 %), and poor Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic risk (25 %).
Several other agents are presently being investigated ([76], summarized in Table 12.3). Recent clinical developments of antibody therapies for use with costimulatory/inhibitory molecules on T cells (immunomodulators) are shown in Fig. 12.2.
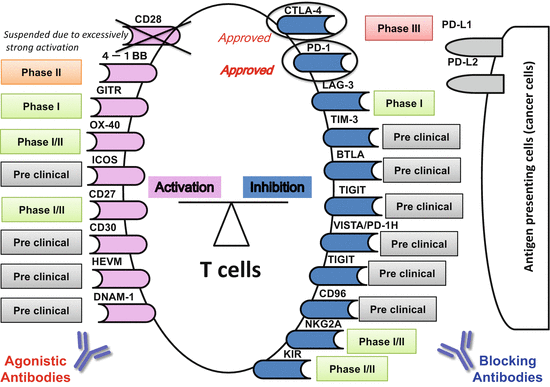

Fig. 12.2
Recent clinical developments of antibody therapies for costimulatory/inhibitory molecules on T cells (immunomodulators)
12.4.3 Combination Therapy
Although immune checkpoint inhibitors have opened up a new era in the treatment of advanced RCC, and there are many promising agents under investigation, the accumulating data on the leading drugs suggest that only a limited number (approximately 20 %) of patients can expect long-term survival with monotherapy [45]. The future challenges include exploring combination therapies that can improve antitumor efficacy without increasing toxicity [47–49]. There are numerous possible novel regimens being evaluated in ongoing clinical trials, seeking the optimal pairing, dosing, and administration sequence ([77], summarized in Table 12.4].
Table 12.4
Immune checkpoint blockade in combination therapy for RCC
Target | Agent | Patient setting | Trial ID | Phase | Trial status | N (N planned, if recruiting) | Regimen | Primary end point | Response | Survival | AEs | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTLA-4 | Tremelimumab | ≧ Second line | NCT00372853 | I | Completed | 28 | Trem + suni trem: 6.0 vs. 10.0 vs. 15.0 mg/kg, every 12 weeks Suni: 37.5 mg/kg daily vs. 50 mg/kg, daily for 4 weeks, every 6 weeks | Safety | PR: 43 % | NA | Rapid onset renal failure was common as DLT | [77] |
PD-1 | Nivolumab | ≧ Second line | NCT01968109 | I/IIa | Ongoing (recruiting) | 540 | BMS986016 (anti-LAG-3 monoclonal Ab) ± nivo | Safety/efficacy | NA | NA | NA | |
Pembrolizumab (MK-3475) | ≧ Second line | NCT02178722 | I/II | Ongoing (recruiting) | 374 | Pemb + epacadostat (INCB024360: IDO inhibitor) | Safety/efficacy | NA | NA | NA | ||
First line | NCT02014636 | I/II | Ongoing (recruiting) | 228 | Pemb + pazo Pemb: 1–10 mg/kg Pazo: 400–800 mg/day | Safety/efficacy | NA | NA | NA | |||
First line | NCT02133742 | I | Ongoing (recruiting) | 60 | Pemb + axit Pemb: 2 mg/kg, every 3 weeks, to find the MTD Axit: starting dose of 5 mg and 3 mg BID | Safety
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|