Fig. 14.1
(a) Interaction of TCR and antigen only is insufficient to T-cell activation. Co-stimulator signal with CD80/86 and CD28 leads to adequate activation. (b) CTLA-4 has higher affinity than CD28 and combines with CD80/86. This leads to suppression of T cell
14.3 PD-1/PD-L1 Signal
After the adequate antigen presentation followed by activation, T lymphocyte killed tumor cell in peripheral tissue. However, this process also includes suppression mechanism. Programmed death-1 (PD-1) is one of the CD28 family molecules discovered in 1992 [16]. T lymphocyte expresses this molecule and interaction with its ligand PD-L1 leads to suppressed immune reaction. The deficiency of PD-1 also leads to autoimmune disease. Its ligand, PD-L1, was expressed in antigen-presenting cell and tumor. Tumor cell escapes from immune reaction with PD-1/PD-L1 interaction, resulting in T-cell apoptosis (Fig. 14.2) [17].
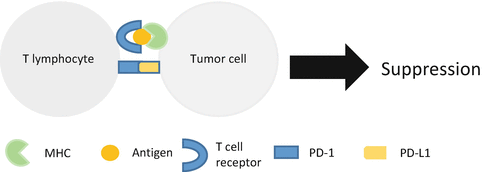

Fig. 14.2
Tumor cell evades immune surveillance by expressing PD-L1. Interaction of PD-1 and PD-L1 leads to T-cell suppression
Tumor sometimes expresses PD-L1, and this leads to poor prognosis in several cancers such as malignant melanoma, esophageal, ovarian, and lung cancer [18–21].
This might be because of immune suppression of tumor in peripheral tissue. Deficiency or inhibition of PD-1 leads to removal of the suppression of immune reaction to tumor. PD-1 knockout mouse or anti-PD-1 antibody showed antitumor efficacy. In malignant melanoma, nivolumab, a fully human IgG4 anti-PD-1 antibody, showed superior efficacy compared with dacarbazine [22]. Median survival time was not reached in nivolumab group and 10.8 months in dacarbazine group. Response rate was 40 % and duration of response was not reached in nivolumab group. This surprising result leads to the enthusiastic investigation of anti-PD-1 therapy.
14.4 Immunotherapy in Lung Cancer
14.4.1 Non-small Cell Lung Cancer
In phase I trial of anti-PD-1 and PD-L1 antibody in patients with advanced cancer, response was observed in 10–18 % of patients with non-small cell lung cancer [23, 24]. PD-1/PD-L1 pathway blockade was expected to have promising effect in non-small cell lung cancer.
In phase II trial for patients with advanced, refractory squamous non-small cell lung cancer, 117 patients received nivolumab [25]. Response rate was 14.5 % (95 % confidence interval [CI], 8.7–22.2). Median progression-free survival was only 1.9 months (95 % CI, 1.8–3.2), but median duration of response was not reached (95 % CI, 8.31; not reached).
In 2015, phase III trial proved the superiority in overall survival of nivolumab compared with docetaxel [26, 27]. In squamous cell carcinoma, 272 patients received nivolumab, at a dose of 3 mg/kg every 2 weeks or docetaxel at a dose of 75 mg/m2 every 3 weeks. Median overall survival was 9.2 months (95% CI, 7.3–13.3) in nivolumab group and 6.0 months (95 % CI, 5.1–7.3) in docetaxel group (hazard ratio [HR] 0.59; 95 % CI, 0.44–0.79) (Table 14.1). The 1-year survival rate was 42 % (95 % CI, 34–50) and 24 % (95 % CI, 17–31). Expression of PD-L1 in tumor cell was not a predictive factor of nivolumab.
Table 14.1
Efficacy of nivolumab compared with docetaxel in squamous cell lung carcinoma
Nivolumab | Docetaxel | HR (95 % CI) | P value | |
|---|---|---|---|---|
Median overall survival (month) (95 % CI) | 9.2 (7.3–13.3) | 6.0 (5.1–7.3) | 0.59 (0.44–0.79) | <0.001 |
1 year survival rate (%) (95 % CI) | 42 (34–50) | 24 (17–31) | ||
Median progression-free survival (month) (95 % CI) | 3.5 (2.1–4.9) | 2.8 (2.1–3.5) | 0.62 (0.47–0.81) | <0.001 |
Median duration of response (month) (range) | Not reached (2.9–20.5) | 8.4 (1.4–15.2) | ||
Response rate (%) (95 % CI) | 20 (14–28) | 9 (5–15) | 0.008 |
In non-squamous cell carcinoma, 582 patients received nivolumab or docetaxel at same schedule with squamous cell carcinoma. Median overall survival was 12.2 months (95 % confidence interval [CI], 9.7–15.0) in nivolumab group and 9.4 months (95 % CI, 8.1–10.7) in docetaxel group (hazard ratio [HR] 0.73; 95 % CI, 0.59–0.89) (Table 14.2). The 1-year survival rate was 51 % (95 % CI, 45–56) and 39 % (95 % CI, 33–45). In this report, although all subgroups favored nivolumab than docetaxel, PD-L1 expression was a strong predictive factor of nivolumab. On the other hand, pembrolizumab, a highly selective humanized monoclonal IgG4-kappa isotype PD-1 antibody, also showed efficacy [28]. In the phase I trial, 495 patients with advanced non-small cell lung cancer received pembrolizumab at a dose of 2 or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks. Objective response rate was 19.4 % and median progression-free survival was 3.7 months. In this study, PD-L1 expression seemed to be a predictive factor of response. Thus, immune checkpoint inhibitor caused a breakthrough in non-small cell lung cancer treatment. Further investigation to improve outcome is warranted.
Table 14.2
Efficacy of nivolumab compared with docetaxel in non-squamous cell lung carcinoma
Nivolumab | Docetaxel | HR (95 % CI) | P value | |
|---|---|---|---|---|
Median overall survival (month) (95 % CI) | 12.2 (9.7–15.0) | 9.4 (8.1–10.7) | 0.73 (0.59–0.89) | 0.002 |
1 year survival rate (%) (95 % CI) | 51 (45–56) | 39 (33–45) | ||
Median progression-free survival (month) (95 % CI) | 2.3 (2.2–3.3) | 4.2 (3.5–4.9) | 0.92 (0.77–1.11) | 0.39 |
Median duration of response (month) (range) | 17.2 (1.8–22.6) | 5.6 (1.2–15.2) | ||
Response rate (%) (95 % CI) | 19 (15–24) | 12 (9–17) | 0.02 |
14.4.2 Small Cell Lung Cancer
In small cell lung cancer (SCLC), the development of immune checkpoint inhibitor is delayed compared with non-small cell lung cancer. In ASCO 2015 in a phase I/II study, nivolumab with or without ipilimumab was reported [29]. Seventy-five patients received nivolumab 3 mg/kg every 2 weeks or nivolumab plus ipilimumab (1 + 1 mg/kg, 1 + 3 mg/kg, or 3 + 1 mg/kg) every 3 weeks for four cycles followed by nivolumab 3 mg/kg every 2 weeks. Overall response rate was 25 and 15 % in nivolumab with or without ipilimumab. On the other hand, in phase Ib trial, 16 PD-L1-positive SCLC patients received pembrolizumab [30]. Response rate was 25 % and durable response was observed.
14.4.3 Adverse Event
The feature of adverse event of immune checkpoint inhibitor is different from cytotoxic agent or molecular target therapy. Although the serious adverse event is rare, it causes immune-related adverse event such as endocrine system disorder. In a phase III trial, the frequency of a serious adverse event with nivolumab was lower than docetaxel (7 % vs. 55 %) [26]. Hematologic toxicity, which is one of the general adverse events in cytotoxic agent, is very low. Anemia and neutropenia occurred only 2 and 1 %. On the other hand, hypothyroidism and pneumonitis occurred in 4–5 % of patients. Anti-CTLA-4 antibody seems more toxic than anti-PD-1 antibody. In malignant melanoma, the frequency of immune-related adverse event of ipilimumab was 60 % [31]. Grade 3 or 4 event occurred in 10–15 % of patients.
The most common adverse event was diarrhea, which occurred in 27–31 % of patients. And endocrine disorder occurred in 3.9–7.6 % of patients. This adverse event occurs not only in the early phase of treatment but in the late phase and requires drug withdrawal or immunosuppressive therapy such as steroid and antitumor necrosis factor α-antibody.
14.4.4 The Feature of Response
The response of immune checkpoint inhibitor has some feature different from conventional chemotherapy. First, delayed response was often observed. Median time to response was 2.2 months (95 % CI, 1.6–11.8) [26]. This occurred even after the discontinuation of treatment. Second, tumor reduction after once tumor progression, called pseudo-progression, was observed. It is difficult to distinguish pseudo-progression from true progression. Then, new response criteria called irRECIST were put forward [32]. Validation of response criteria is warranted.
14.4.5 Predictive Factor
The feature of immunotherapy seemed to be that specific population showed efficacy and durable response. Then, it is crucial to investigate the predictive factor such as EGFR mutation for EGFR-TKI therapy.
The most expected simple answer for anti-PD-1 or anti-PD-L1 therapy is expression of PD-L1 in tumor cell. In non-squamous cell lung cancer treated with nivolumab and non-small cell lung cancer treated with pembrolizumab, expression of PD-L1 seemed to be a predictive biomarker [27, 28]. However, in squamous cell lung cancer treated with nivolumab, it was not a predictive factor [26]. And there was some problem for the estimation of expression of PD-L1. First, the most adequate antibody to estimate the expression of PD-L1 was unclear. In nivolumab study, anti-PD-L1 antibody clone 28-8 (Dako, North America) was used. And anti-PD-L1 antibody clone 22C3 (Merck) was used in pembrolizumab study. It is unknown which antibody is suitable for the estimation of PD-L1. Second, the cutoff value of PD-L1-positive tumor cell was also unclear. Third, expression of PD-L1 might be influenced by previous chemotherapy, molecular target therapy, and radiotherapy. It is also unknown how these treatments influence the expression of PD-L1. In nivolumab study, PD-L1 was estimated by archival samples in some cases. It might be suitable to estimate the samples obtained just before anti-PD-1 antibody treatment. However, there exists heterogeneity of PD-L1 expression. Ilie et al. reported that expression of PD-L1 might be underestimated in biopsy sample, and there was poor association between biopsy samples and surgically resected sample [33]. These factors implicated whether PD-L1 expression in tumor cell is a predictive factor in anti-PD-1 therapy. It is desirable to establish the adequate method of estimation of PD-L1 expression. The difference of estimation of PD-L1 was shown in Table 14.3. And patients with PD-L1-negative tumor were also observed for response. It may be difficult to distinguish responder from nonresponder with PD-L1 expression alone. On the other hand, anti-PD-1 antibody is the most sensitive in several cancers such as malignant melanoma and non-small cell lung cancer which has higher mutational burden than other cancers. Then, other promising predictive factor is mutational burden of tumor. Rizvi et al. investigated the whole genome of tumor that received pembrolizumab [34]. They reported that tumors with higher nonsynonymous mutational burden, molecular smoking signature, and DNA repair pathway mutation showed good correlation with high response rate and progression-free survival of pembrolizumab. This result is consistent with ipilimumab in malignant melanoma [35]. In other cancers, mismatch repair deficiency predicted the response of immune checkpoint inhibitor [36]. Nonsynonymous mutational burden was correlated with neoantigen burden and might lead to good response of T cell. However, it might be difficult to identify crucial predictive factor such as EGFR mutation in EGFR-TKI therapy in the case of immune checkpoint inhibitor because immune reaction required a complicated process. Not only tumor factors but also host factors such as dendritic cell, T lymphocyte, and their activation process participate in the efficacy of immunotherapy.
Table 14.3




Difference of evaluation of PD-L1 expression in two studies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree