Fig. 23.1
Structure of 5T4 molecules. Human and mouse 5T4 analyzed by a homology modeling approach using the variable lymphocyte receptor A29 (PDB entry 2o6q) and energy minimized to produce RAW structures (Courtesy of Alex Weber and Andriy Kubarenko, DKFZ, Germany)
These expression patterns and mechanistic studies supported the use of 5T4 as a suitable target for several different types of immunotherapy. More recently, further insights into the function of 5T4 in modulating cancer spread have been established.
23.2 5T4 and Epithelial Mesenchymal Transition (EMT)
EMT occurs during embryonic development and is important for the metastatic spread of epithelial tumors [26]. The 5T4 oncofetal antigen is an early marker of differentiation of mouse and human embryonic stem (ES) cell [27–29]. This process is also an EMT-like event characterized by the differentiation of ES cells in monolayer culture associated with an E- to N-cadherin switch, upregulation of E-cadherin repressor molecules (Snail and Slug proteins), and increased matrix metalloproteinase (MMP-2 and MMP-9) activity and motility [30, 31]. Interestingly, undifferentiated E-cadherin KO ES cells constitutively express surface 5T4, while abrogation of E-cadherin-mediated cell-cell contact in undifferentiated ES cells using neutralizing antibodies results in increased motility, altered actin cytoskeleton arrangement, and a mesenchymal phenotype with cell surface expression of 5T4 molecules [30, 31]. These data and our previous observations showing 5T4 overexpression in epithelial cells associated with downregulation of E-cadherin [22] suggest that the latter functions to prevent cell surface localization of 5T4 possibly by stabilizing cortical actin cytoskeletal organization.
23.3 5T4 Modulation of Chemokine and Wnt Signaling Pathways
To further investigate additional changes on early ES differentiation, a comparative microarray analysis of undifferentiated (5T4 –ve) and early differentiating (5T4 +ve) murine ES cells was performed. One particular transcriptional change identified was the downregulation of transcripts for the dipeptidyl peptidase IV, CD26, which codes for a cell surface protease that cleaves the chemokine CXCL12 [32]. CXCL12 binds to the widely expressed cell surface seven transmembrane domain G-protein-coupled receptor CXCR4 [33] and to the recently identified receptor CXCR7/RDC1 [34]. Subsequently, 5T4 molecules were shown to be required for functional expression of CXCR4 at the cell surface in some embryonic and tumor cells [17, 35, 36]. Both CXCL12 expression and CXCR4 expression have been associated with tumorigenesis in many cancers including breast, ovarian, renal, prostate, and neuroblastoma [33, 37, 38]. These CXCR4-expressing tumors preferentially spread to tissues that highly express CXCL12, including the lungs, liver, lymph nodes, and bone marrow. The observation that some mAbs against m5T4 can inhibit CXCL12 chemotaxis of differentiating ES cells and mouse embryo fibroblasts (MEF) suggests a 5T4 contribution at the cell surface facilitating the biological response to CXCL12 through CXCR4. It is apparent that 5T4 is not a simple chaperone providing for trafficking of the receptor to the cell surface since CXCR4 surface expression depends on microtubules, whereas 5T4 does not [35]. Further, FRET studies do not support a direct interaction between the molecules, while preliminary proteomic analysis following cross-linking of 5T4 molecules indicates many cytoskeleton-associated interactions [39] (Vaghjani and Stern, 2012). This regulation of CXCR4 surface expression by 5T4 molecules provides a novel means to control responses to the chemokine CXCL12, for example, during embryogenesis, but can also be selected to advantage the spread of a 5T4-positive tumor from its primary site. We have recently shown that 5T4 also inhibits Wnt/β-catenin canonical while concomitantly activating the non-canonical Wnt signaling pathway associated with increased motility [40]. It is likely that the integrated 5T4 regulation of both the chemokine and Wnt pathways acts to promote cancer spread as well as functional migration in development (Fig. 23.2).
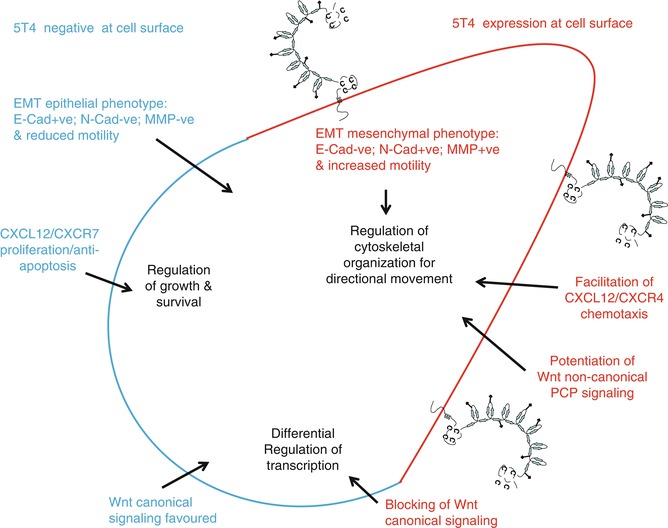

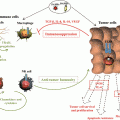
Fig. 23.2
5T4 functional influences on tumor spread. Integrated 5T4 regulation of both the chemokine and Wnt pathways acts to promote cancer spread as well as functional migration in development and cancer
23.4 Vaccines
The selective tumor expression pattern of 5T4 as well as a putative role in tumor spread established this target as a suitable target for vaccine immunotherapy. Viral vector-based immunotherapy aims to overcome the relative poor immunogenicity of TAAs by presenting the antigens in a foreign viral vector with the principal goal of generating effector T cells able to kill 5T4-positive tumors. Lack of high-avidity T-cell receptors (TCRs) in the T-cell repertoire and specific or nonspecific T regulatory cells may be major limiting factors for vaccine immunogenicity and effectiveness. The highly attenuated and modified vaccinia virus ankara (MVA) strain was the viral vector of choice for expressing either human or mouse 5T4 and evaluation of immunogenicity and antitumor activity in preclinical studies.
23.4.1 Preclinical Studies
Immunization of mice with MVA-h5T4 and MVA-m5T4 constructs induced antibody responses to human and mouse 5T4, respectively. Mice vaccinated with MVA-h5T4 were protected when challenged with syngeneic tumor line transfectants expressing h5T4. In active treatment studies, inoculation with MVA-h5T4 was able to treat established CT26-h5T4 lung tumor and to a lesser extent B16.h5T4 subcutaneous tumors [41]. In this xenogeneic-TAA model, it was shown that the likely component of protection was antibody with induction dependent on the CD4+ T cells [42]. Vaccination of mice with MVA-m5T4, a perhaps more relevant model for human cancers, was able to control the growth of autologous B16 cells expressing m5T4 in a tumor protection scenario. Furthermore, mice vaccinated with MVA-m5T4 showed no signs of autoimmune toxicity [41].
Further studies investigated the human T-cell repertoire. Human CD8+ T-cells recognizing HLA-restricted 5T4 peptides have been identified by methods using monocyte-derived dendritic cells (DC) to stimulate peripheral blood lymphocytes from healthy individuals in the absence of CD4+ T cells [43, 44]. These data are consistent with the influence of Tregs on limiting immune responses to TAA [45]. Subsequently it was shown that the generation of CD4+ cells recognizing 5T4 peptides also required initial depletion of T regulatory cells. Interestingly, CD4+ T cells spontaneously recognizing a 5T4 epitope restricted by HLA-DR were identified in tumor-infiltrating lymphocytes from a regressing renal cell carcinoma (RCC) lung metastasis. These cells produced both interferon-gamma (IFN-γ) and IL-10 suggesting that such h5T4-specific CD4+ T cells boosted or induced by vaccination could act to modulate both cell- or antibody-mediated antitumor response either positively or negatively depending on the differentiation status of the T cell [46].
23.4.2 Early-Phase Clinical Trials of MVA-h5T4 (TroVax)
The preclinical data supported the development of TroVax for tumor immunotherapy. A succession of phase I or II clinical trials in colorectal, prostate, and RCC patients (including with chemotherapy or cytokine treatments) established the optimal dose and route of vaccination as well as safety, tolerability, and vaccine immunogenicity (serology, lymphocyte proliferation, and ELISPOT assays). Two or three TroVax immunizations were needed to generate somewhat transient 5T4-specific cellular immunity, and this was independent of the vector-specific response leading to a protocol of multiple booster vaccinations. In several trials there was evidence of association of 5T4 immune responses with better clinical outcome albeit in relatively small study sizes (summarized in Table 23.1). For example, in a clinical trial of TroVax in patients undergoing surgical resection of colorectal cancer liver metastases, 17 of 19 colorectal cancer patients showed 5T4 expression in the liver metastases or surrounding stroma and 18 mounted a 5T4-specific cellular and/or humoral response. In patients who received at least four vaccinations and potentially curative surgery (n = 15), those with above median 5T4-specific proliferative responses or T-cell infiltration into the resected tumor showed significantly longer survival compared with those with below median responses [49]. Further investigations assessed the levels of systemic T regulatory cells, plasma cytokine levels, phenotype of tumor-infiltrating lymphocytes including T regulatory cells (Tregs), and tumor HLA class I loss of expression. More than half of the patients showed phenotypes consistent with relative immune suppression and/or escape, highlighting the complexity of positive and negative factors challenging any simple correlation with clinical outcome [53].
Table 23.1
TroVax: early clinical studies of immunogenicity and clinical response
Indication trial (patients) | Patient treatment regime | % 5T4 specific immune response (IR) | Immune and clinical responses (patients with IR measures) | Reference | |||
|---|---|---|---|---|---|---|---|
Antibody | Proliferation | ELISPOT | Total | ||||
Metastatic colorectal phase 1 (22) | Post chemotherapy | 82 | 88 | 100 | 94 | Antibody vs. TTP/survival (17) | Harrop et al. [42] |
Metastatic colorectal phase II (19) | 1st line+5FU/LV/irinotecan | 83 | 83 | 92 | 100 | None (12) | Harrop et al. [47] |
Metastatic colorectal phase II (17) | 1st line+5FU/LV/ oxaliplatin | 91 | 91 | 91 | 100 | ELISPOT vs. tumor response (11) | Harrop et al. [48] |
Metastatic colorectal phase II (20) | Adjuvant to liver metastasis surgery | 100 | 88 | 53 | 100 | Proliferation vs. survival (17) | Elkord et al. [49] |
Prostate- hormone refractory phase 11 (27) | 2nd line+/−GM-CSF | 100 | nt | 36 | 100 | ELISPOT vs. PFS (24) | Amato et al. [48] |
Metastatic renal cell carcinoma phase II (11) | 1st & 2nd line + IFN-α | 100 | nt | 36 | 100 | None (11) | Hawkins et al. [50] |
Metastatic renal cell carcinoma phase II (28) | 1st & 2nd line +/−IFN-α | 91 | nt | 30 | 91 | Antibody vs. survival (23) | Amato et al. [51] |
Metastatic renal cell carcinoma phase II (25) | 2nd line low dose Il-2 | 90 | nt | 30 | 90 | ELISPOT vs. survival (20) | Amato et al. [50] |
Metastatic renal cell carcinoma phase II (28) | 2nd-line high-dose IL-2 | 100 | nt | 36 | 100 | Antibody vs. survival (19) | Kaufman et al. [52] |
23.4.3 TroVax Phase III Clinical Trial in RCC
Building on the several phase II studies in RCC (Table 2 in ref. [54]), a phase III trial in RCC patients was designed to determine if the addition of TroVax to available standard of care (SOC) therapy could improve survival for patients with metastatic RCC. This international multicenter trial randomized 733 patients who received seven or eight injections of TroVax (n = 365) or placebo (n = 368) along with either interferon-α (IFN-α), IL-2, or sunitinib as first-line treatment [55]. The primary endpoint was overall survival, and progress free survival, objective response rate, and safety were secondary measures. When the survival data was censored, there was a median follow-up of 12.9 months. While TroVax was safe and well tolerated in all these patients, it failed to meet its primary endpoint, as there was no significant difference in survival for the TroVax- and placebo-treated groups. However, in the subset of patients with a good prognosis (Motzer grade 0) receiving IL-2, there was a significantly improved survival with TroVax compared to the placebo group. No other SOC subset, albeit less mature, showed evidence of a TroVax benefit. Analysis of a selected group of 50 TroVax vaccinated patients with the highest increase in 5T4 antibody responses showed a favorable survival compared to placebo patients, while a similar group with the highest increase in MVA antibody did not.
5T4 antibody response was quantified after the third and fourth vaccinations, and an immune response surrogate (IRS) was constructed and then used to evaluate survival benefit in 590 patients from the phase III study. A high antibody response was associated with longer survival within the TroVax-treated group. The IRS was derivative from a linear combination of pretreatment 5T4 antibody levels, hemoglobin, and hematocrit and was able to predict patient benefit in the phase III study. Importantly, the IRS was associated with antibody response and survival in independent data sets from other TroVax trials [56, 57]. Further statistical modeling identified several baseline clinical factors associated with inflammatory anemia (CRP, hemoglobin, hematocrit, IL-6, ferritin, platelets), which demonstrated a significant relationship with tumor burden and survival. From these prognostic factors, the mean corpuscular hemoglobin concentration (MCHC) was shown to be the best predictor of treatment benefit and was positively associated with tumor shrinkage in different clinical studies of TroVax in vaccinated patients. These results support a view that patients with a relatively small tumor burden and high MCHC would be most likely to benefit from Trovax vaccination [58]. However, our studies in colorectal cancer patients with liver metastasis highlighted a multiplicity of immune regulatory factors that can negatively influence the outcome of patients even with effective immunogenicity of the vaccine [46, 53].
TroVax has now been tested in over 500 patients in ten different clinical trials, and in most patients antibody responses are induced, whereas cellular T-cell responses are less frequently detected (reviewed in Kim et al. [54]). A desired goal of vaccination is the generation of 5T4 effector CD8+ T cells although the most frequently used T-cell assay was proliferation which probably reflects a CD4 response. Only relatively rarely have high-frequency CD8+ T-cell responses been definitively demonstrated by ELISPOT. The available evidence from the TroVax clinical studies has suggested that the use of the same vaccine for priming and multiple boosting does not limit the 5T4 immune response as a result of anti-vector responses. However, preclinical studies of different prime/heterologous boost vaccine combinations (replication defective adenovirus (rAd) and retrovirally transduced DC lines expressing h5T4) have shown that the order of immunization can influence the overall therapeutic efficacy by the generation of different 5T4-specific cellular immune responses in tumor-bearing mice [59]. In particular, a role for Tregs in limiting the therapeutic value of vaccination was demonstrated. The use of the complete 5T4 coding sequence in the vaccine construct could provide epitopes able to both stimulate regulatory as well as effector T-cell responses.
23.4.4 Insights from the 5T4 KO Mouse
A recent study exploited the 5T4 knockout (KO) mice to analyze the mechanisms by which endogenous expression of 5T4 influences autologous T-cell immunity and tolerance [60]. While the 5T4 KO mice show no obvious changes in T-cell, B-cell, and/or myeloid populations, 5T4 is expressed in murine thymus and thus might influence the repertoire and/or induction of specific Tregs cells leading to the control of natural or vaccine-induced immunity [61]. Mouse 5T4-specific T-cell epitopes were identified using the 5T4KO mouse, and wild-type (WT) responses were evaluated as a model to refine and improve immunogenicity. Studying the immune response (INF-γ ELISPOT) of 5T4KO mice to rAdm5T4 vaccination identified only two dominant H2b-restricted epitopes for which the WT mouse response was either significantly reduced (only low-avidity CD8) or absent (CD4). Other data suggest the possibility that in the absence of WT 5T4-specific CD4+ T helper cells, there is an alternative differentiation process generating 5T4-specific Tregs. While a single rAdm5T4 vaccination of 5T4KO mice provides protection against B16m5T4 tumor challenge, there is no effect in WT mice. Treatment of WT mice with folate receptor 4 (FR4) antibody to deplete Tregs [62], after Adm5T4 vaccination, alters the balance of effectors and provides a modest protection against autologous B16m5T4 challenge. These data are consistent with the efficacy of 5T4 and some other TAA vaccines being limited by the combination of TAA-specific Tregs, as well as the deletion and/or alternative differentiation of CD4+ and/or CD8+ T cells [60]. An alternative to vaccination is the adoptive transfer of tumor-specific lymphocytes. To test the potency of this approach in the m5T4 model, primed 5T4KO splenocytes were adoptively transferred to naïve WT recipient animals, but failed to protect against B16m5T4 tumor challenge. Attempts to in vivo modulate Tregs using FR4 mAb were unsuccessful in achieving major protection against tumor challenge despite the clear evidence of survival of adoptively transferred T cells. Protocols for clinical adoptive cell therapy now incorporate pre-conditioning which results in a reduction of suppressor cells and conditions which favor homeostatic expansion [45, 63, 64]. However, a clinical study investigating the adoptive transfer of CD25-depleted (includes Tregs) peripheral blood mononuclear cells in cyclophosphamide/fludarabine pre-conditioned RCC patients showed that this treatment resulted in only a short period of in vivo Tregs depletion [65].
23.4.5 Improving Vaccine Regimens
The challenge for optimizing 5T4 (and other TAA) vaccine immunogenicity requires a means to stimulate appropriate effector T-cell responses and not concomitantly immunomodulatory cells which may always limit the therapeutic effect. We are exploring the use of 5T4-specific CD8 epitopes engineered into an Immunobody DNA as this approach [66] can potentially improve vaccine immunogenicity by favoring generation of high-avidity CD8+ T cells capable of functioning in an autologous tumor-bearing animals.
Another way to overcome limited immunity to TAA is by modulating costimulatory/inhibitory signals on T cells such as through CTLA-4 [67]. However, while clinical results with the humanized anti-CTLA-4 antibody, ipilimumab, have led to its licensing for the treatment of advanced melanoma; the precise mechanism accounting the effects in patients is not known [68]. The benefits of increased survival of a few months are only seen in a small subset of patients. Indeed, in these terms a study of the Pfizer CTLA-4 antibody, tremelimumab, in 18 patients with metastatic gastric and esophageal adenocarcinomas as a second-line treatment also gave encouraging results [69]. Four patients had stable disease with clinical benefit, and one patient achieved a partial response after eight cycles (25.4 months) and remained well at 32.7 months. Interestingly, de novo proliferative responses to 5T4 (8 of 18 patients) and carcinoembryonic antigen (5 of 13) were detected. Indeed, patients with a posttreatment carcinoembryonic antigen proliferative response had a median survival of 17.1 months compared with 4.7 months for nonresponders. Such in vitro evidence of enhanced proliferative responses to relevant TAAs suggests that combining CTLA-4 blockade with specific vaccination may provide additional benefit [69].
23.5 5T4 Antibody-Targeted Superantigen Therapy
Bacterial superantigens such as staphylococcal enterotoxin A (SEA) can activate T cells by linking the latter through binding to a particular family of V-beta chain containing TCRs to MHC class II molecules on antigen-presenting cells. With an antibody-superantigen fusion protein, large amounts of cytotoxic and cytokine-producing T cells can be targeted by the antibody specificity for a TAA for in vivo tumor treatment [70, 71]. Challenges in developing safe and efficacious therapy for cancer depend on selection of a suitable TAA, overcoming the toxicity associated with MHC class II binding, and any preexisting immunity to the bacterial protein [72].
23.5.1 Preclinical Studies
A first-generation 5T4 mAb-derived Fab-SEA fusion (ABR-214936) incorporated a point mutation in the SEA sequence reducing the affinity for binding to MHC class II molecules and optimized for bacterial production [73]. This agent (ABR-214936) maintained 5T4-specific superantigen antibody-dependent cellular cytotoxicity (SADCC) while toxicity for MHC class II expressing cells was reduced by 1,000-folds in vitro (SDCC); therapeutic efficacy was demonstrated in murine xenograft tumor models [74].
23.5.2 Early-Phase Clinical Studies
In a phase I study in non-small cell lung carcinoma (NSCLC) patients, a maximum tolerated dose (MTD), given intravenously over 4 days, as a function of the preexisting anti-SEA antibody was determined [75]. In phase II studies of ABR-214936 in RCC patients, the treatment cycle was repeated after 1 month and survival was significantly prolonged compared to that expected. Patients receiving higher drug exposure had greater disease control and lived almost twice as long as expected, whereas low drug exposure patients survived as expected (Fig. 23.3); sustained IL-2 production at day 2 appeared to be a biomarker for the clinical effect [76].
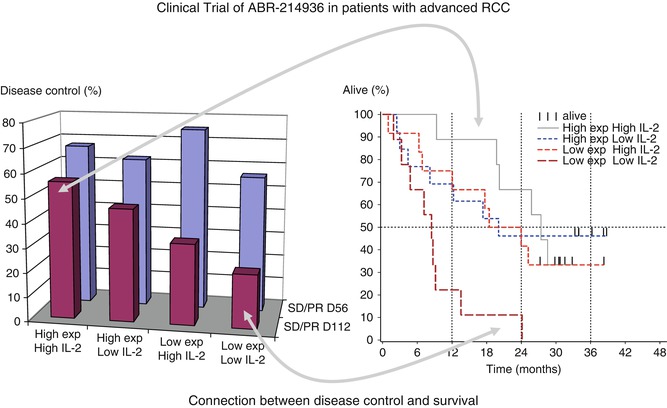

Fig. 23.3
5T4 antibody-directed superantigen therapy. Clinical trial of 5T4 antibody-directed superantigen therapy in patients with advanced RCC. This shows that patients with high IL-2 after the second infusion and high exposure are more likely to have disease control at day 112 and the longest survival (Adapted by permission from British Journal of Cancer: Shaw et al. [76])
The high degree of disease control and the prolonged survival suggested this treatment could be effective and led to the development of an improved variant (ANYARA or naptumomab estafenatox or ABR-217620). This version has 90 % homology to ABR-214936, incorporating a hybrid SEA/E-120 superantigen sequence with additional point mutations reducing MHC class II binding and antigenicity [77, 78]. Preclinical evaluation showed reduced binding to preformed anti-superantigen antibodies, lower toxicity, higher affinity for 5T4, and improved tumor cell killing. Phase I clinical studies showed that ANYARA was well tolerated both as monotherapy and in combination with docetaxel, and there was a good correlation of the preclinical studies with the MTD [79]. Evidence of immunological and antitumor activity included a dose-dependent induction of IL-2 and INF-γ (biomarkers for T-cell activation), selective expansion of ANYARA reactive T cells, infiltration of T cells into the tumor, plus selective retention of ANYARA in tumor tissue as demonstrated using PET.
23.5.3 A Phase II/III Clinical Trial in RCC
A multinational (50 sites in Europe: UK, Russia, Ukraine, Bulgaria, Romania), randomized phase II/III study of ANYARA in combination with IFN-α vs. IFN-α alone in 513 advanced RCC patients has been conducted. The safety profile was good and in line with previous observations; the most common adverse events associated with ANYARA treatment were grade 1–2 fever, nausea, and vomiting. No new and unexpected safety concerns were identified in the study. Unfortunately, the primary endpoint – to show a survival advantage in the intention to treat population – was not reached. Unexpectedly, and in contrast to previous studies conducted in other countries, a majority of the patients showed high levels of preformed antibodies against the superantigen component of ANYARA. A subgroup analysis, excluding patients with high levels of preformed antibodies, resulted in a trend for survival benefit with ANYARA treatment. This was consistent with the results of the previous version ABR214936 in RCC patients [76]. Interestingly, high baseline levels of IL-6 were associated with a poorer outcome in this study, and this was also seen in trials of RCC patients treated with TroVax [58] or pazopanib [80]. In a hypothesis-generating analysis of approximately 25 % of patients with low/normal levels of base line IL-6 and low anti-superantigen antibody levels, a statistically significant treatment advantage for overall survival was seen (p = 0.02, HR = 0.59). In North America and Western Europe, this subgroup accounts for 40–50 % of the total number of advanced RCC patients [81]. Additional analyses of the ANYARA phase II/III study data are ongoing with future development strategies aiming at a pivotal phase II/III study with ANYARA in combination with a tyrosine kinase inhibitor in the favorable RCC subgroup.
23.6 Other 5T4 Antibody-Targeted Therapies
This section will consider therapies using 5T4 antibody for the delivery of toxins and inhibition of function in cancer spread and in the context of chimeric antigen receptors expressed in T cells using retroviruses.
23.6.1 Antibody-Drug Conjugates (ADC)
ADCs chemically combine the specificity of the antibody with a cytotoxic drug. The challenge is to produce an efficacious and safe agent, and this demands optimizing the properties of a suitable TAA-specific antibody in combination with the linkage chemistry and the payload characteristics. The original mAb 5T4 (clone H8) was shown to internalize into cells and utilized to target the calicheamicin toxin. The latter is a potent cytotoxic drug which causes double-strand DNA breaks. The conjugation methodology used stable chemical linkers between antibody and drug which restricted the release of calicheamicin to cells that internalize the ADC. The efficacy of the anti-5T4 conjugates was demonstrated in several tumor models including an orthotopic model for 5T4-positive lung cancer [82]. Another study showed that 5T4 is expressed on tumor-initiating cells (TICs) in (NSCLC) xenografts, and this correlated with worse clinical outcome for the patients [16]. Consistent with other mechanistic studies [30, 31], co-expression of 5T4 and factors involved in the epithelial-to-mesenchymal transition was observed in undifferentiated but not in differentiated lung tumor cells.
These observations support the possibility that the anti-5T4 ADC might cause complete regression of tumors through targeting 5T4-expressing TICs, even where there is considerable heterogeneity in expression of 5T4 within the tumor. To test this, the efficacy of an anti-5T4 ADC on the growth of two patient-derived xenograft (PDX) lines with heterogeneous and different levels of 5T4 expression predominantly at the lung tumor-stroma interface was assessed. These tumors were treated with anti-5T4 ADC, anti-CD33 ADC, or vehicle; the anti-CD33 ADC served as a negative control because these PDX lines do not express CD33. In both cases, treatment with anti-5T4 ADC caused tumor regression, and no regrowth was observed even 3 months after the last dose; in contrast, treatment with anti-CD33 ADC or vehicle did not inhibit tumor growth. Treatment with calicheamicin (not conjugated to an antibody) did not show any significant impact on tumor growth. In contrast to the efficacy observed with anti-5T4 ADC, treatment of both PDXs with cisplatin at the maximum tolerable dose regressed tumors only transiently, and the tumors regrew after treatment was completed. These results highlight the superior long-term efficacy of an ADC that targets TICs as compared with a conventional chemotherapeutic. Thus, despite heterogeneous expression of 5T4 in NSCLC patient-derived xenografts, treatment with an anti-5T4 antibody-drug conjugate resulted in complete and sustained tumor regression. Thus, the aggressive growth of heterogeneous solid tumors can be blocked by therapeutic agents that target a subpopulation of cells near the top of the cellular hierarchy [16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree