Laboratory evidence and clinical evidence suggest that some patients with myelodysplastic syndrome (MDS) have immunologically mediated disease. This article describes the laboratory evidence supporting a role for the immune system in the marrow failure of MDS and clinical trials using IST in these patients.
Laboratory evidence and clinical evidence suggest that some patients with myelodysplastic syndrome (MDS) have immunologically mediated disease. Autoimmune conditions such as Reynaud syndrome, lupus, rheumatoid arthritis, and polymyalgia are more frequent in MDS patients, and activated cytotoxic T cells and elevated levels of tumor necrosis factor alpha (TNF-α) and interferon γ (IFNγ) are found in MDS. Cytokine dysregulation and excessive apoptosis are most notable in early MDS, where up–regulation of Fas-receptor and Fas-ligand expression are hallmarks of the disease.
The concept of using immunosuppressive treatment (IST) for bone marrow (BM) failure originates severe aplastic anemia (SAA) patients. Gluckman and colleagues were the first to show that hematopoietic recovery could occur in SAA following antithymocyte globulin (ATG) treatment alone. As experience and success in IST for SAA accumulated, small numbers of patients with BM failure secondary tp hypoplastic MDS were treated successfully. The response of such patients led to the establishment a clinical trial of IST at the National Institutes of Health in 1994 to evaluate the ability of ATG to improve BM failure in all forms of MDS.
This study showed improvement in 30% of patients with MDS after ATG. Further efforts to identify the profile the characteristics of the IST responder have resulted in better response rates to IST. This article describes the laboratory evidence supporting a role for the immune system in the marrow failure of MDS and clinical trials using IST in these patients.
Cytokine dysregulation
TNFα is the cytokine most implicated in the pathophysiology of MDS. TNF is overexpressed in cultured cells from patients with MDS. This cytokine is elevated in marrow biopsies and in plasma. TNF-related apoptosis-inducing ligand (TRAIL) and IFNγ have been reported elevated in peripheral blood lymphocytes from patients with MDS. TNF production correlated loosely with disease subtype, being most prominent in patients with low-risk MDS. Some investigators have suggested that the source of TNF, and perhaps other negative regulators, may be macrophages or other cells located within BM stroma. Both TNF and IFNγ up-regulate Fas expression on the CD34 cell and trigger apoptosis. TRAIL is a member of the TNF family, which controls apoptosis by binding to agonistic receptors 1 and 2 (TRAIL-R1, TRAIL-R2) and decoy receptors 3 and 4 (TRAIL-R3, TRAIL-R4). Although TRAIL is present in negligible amounts in normal marrow, it is constitutively expressed in MDS marrow, where cells are more sensitive to its cytotoxic and inhibitory effects. Interestingly, TRAIL produced apoptosis selectively in cytogenetically abnormal cells, identified by fluorescence situ hybridization markers. These findings were attributed to up-regulation of TRAIL-receptors 1 and 2 on the aneuploid clone and to differential expression (or function) of intracellular inhibitors of apoptosis Fas-associated death domain-like interleukin (IL)-1β-converting enzyme inhibitory protein (FLIP). FLIP is important in controlling apoptosis in normal cells. Isoforms of FLIP, which result from alternative splicing of mRNA, may have either proapoptotic or antiapoptotic properties depending on their length and may regulate apoptosis in normal marrow. In early MDS, when apoptosis is most prominent, the antiapoptotic long isoform of FLIP (FLIP L ) is down-regulated.
Model for immunologically mediated marrow destruction
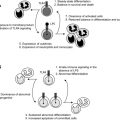
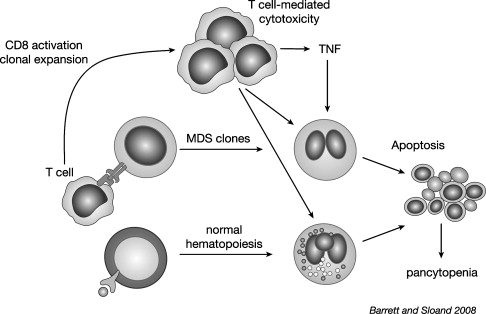
Early MDS is characterized by an increased apoptosis in marrow cells. It is not clear whether programmed cell death is related to alteration in genetic programming or is a result of an immune attack. Evidence that the ineffective hematopoiesis is immunologically mediated comes from experiments showing suppression of hematopoiesis by lymphocytes derived from patients with active disease, but not following successful treatment with ATG. Normally, quiescent lymphocyte populations contain a diverse distribution of thousands of individual clones within 23 T cell receptor (TCR) Vβ families. During an immune response, expansion of T cell clones specific for the corresponding antigen occurs. This expansion may be detected by flow cytometry using antibodies directed against the TCR Vβ subfamilies, or more specifically by spectratyping or CDR3 length analysis. Like patients with multiple sclerosis, those receiving vaccinations, and patients with infections, expanded Vβ subfamilies where they appear to be cytotoxic to hematopoietic cells present in some cases of MDS.
The mechanism for immunologically mediated marrow damage in MDS has been defined most clearly in patients presenting with trisomy 8 as a single karyotypic abnormality. The authors chose trisomy 8 to investigate, because such patients are likely to respond to IST. Additionally, the dysplastic clone can be identified readily by FISH. Initially, the authors showed that early apoptotic changes appeared to be restricted to the trisomy 8 clone, because flow cytometric-sorted Fas-positive, annexin-positive cells were enriched in, or entirely composed of trisomy 8 cells. T cells from expanded Vβ families selectively killed trisomy 8 cells in vitro, indicating that T cells could target trisomy 8 cells specifically. Consistent with this finding was the observation that depletion of cytotoxic T cells in BM cell improved growth of trisomy 8 colonies. Despite such specificity, it remained a possibility that normal BM cells could be killed as innocent bystanders by TNF and IFN-γ elaborated by activated T cells. Trisomy 8 cells survive despite showing signs of early apoptosis; DNA degradation did not develop, and cell death did not occur. The resistance of trisomy 8 cells to apoptotic cell death might be attributed to up-regulation of c-myc present on chromosome 8, and survivin, which may be responsible for resistance of trisomy 8 cells to T cell attack. Indeed, when these proteins are knocked down or inhibited, trisomy 8 cells generally die in vitro.
It may be hypothesized that CD8+ T cells recognize a neoantigen or an overexpressed self-antigen presented by major histocompatability locus (MHC) class 1 molecules on trisomy 8 cells. The authors searched for candidate antigens by examining microarray data on CD34 cells obtained from MDS patients with trisomy 8 and other cytogenetic abnormalities. Wilms tumor protein 1 (WT-1) was markedly overexpressed in trisomy 8 cells. WT-1 is a zinc finger transcription factor located on chromosome 11p13 and was implicated in the pathogenesis of Wilm tumor. WT1 is expressed at low levels in normal tissues, including kidney, ovary, testis, and spleen, but not in normal BM or peripheral blood (PB). It is overexpressed in various hematological malignancies including leukemia, MDS and paroxysmal nocturnal hemoglobinuria (PNH). Cytotoxic T cells recognize a number of epitopes from the WT1 protein.
In MDS, WT1 transcript levels increase with disease progression and correlate with the International Prognostic Scoring System (IPSS) stage. In the authors’ studies, WT1 m-RNA and protein were increased in many IPSS-intermediate 1 (int-1) MDS patients, but were highest in patients with trisomy 8. CD8+ T cells from patients who ultimately became IST responders generated TNF-α and IL-2 in response to WT1 peptide stimulation. CD8 cytotoxic T cells, recognizing WT1 126 peptide, also could be detected by tetramer analysis in HLA-A*0201-positive patients. These findings do not exclude the possibility that other antigens on MDS cells are recognized by the immune system, and that their identification will require further testing of peptide libraries. In this regard, PR1, a peptide derived from myeloid-restricted azurophil granule proteins, can provoke T cell responses in patients with MDS. In summary, therefore, the authors’ studies showed that MDS cells could overexpress tumor-specific antigens that are responsible for inducing autologous T cell cytotoxic T cell responses against the MDS lineage. In the case of trisomy 8, however, the MDS target cells are relatively resistant to such T cell-media apoptosis. Fig. 1 outlines the authors’ working model of T cell interactions with trisomy 8 cells that may be extrapolated to other MDS patient responsive to IST.
Patients with trisomy 8 have a high probability of responding to IST. Curiously, the authors found that selective patients may have normal bone marrow cytology despite the persistence of this karyotypic abnormally with trisomy 8 who recover their blood counts after IST to normal marrow morphology yet do not lose the trisomy 8 populations in the marrow. Indeed the percentage of trisomy 8 positive cells by FISH in blood and marrow can actually increase. Despite the increase in karyotypically abnormal cells, these patients remain stable for years and have shown a very low risk of progressing to acute leukemia. These observations reveal unique and unexpected consequences of the interaction of the immune system with MDS. First, in untreated patients, the survival of the trisomy 8 clone despite in the presence of the T cell response suggests that the MDS cells are adapted to survive T cell attack. Experimental data support this idea; trisomy 8 cells show signs of apoptotic induction with increased caspase and BCL-2 but appear to avoid destruction from T cell-mediated apoptosis by up-regulating survivin. This stalled apoptotic process results in “living dead” cells with dysplastic morphology. Second, the recovery of normal morphology in the trisomy 8 cells after IST strongly suggests that morphologic features of dysplasia at least in trisomy 8 disease may represent cellular responses induced by T cell attack. Lastly, and surprisingly, in the absence of T cell attack the expanded trisomy 8 clone can establish normal hematopoiesis, which remains stable for many years.
Model for immunologically mediated marrow destruction
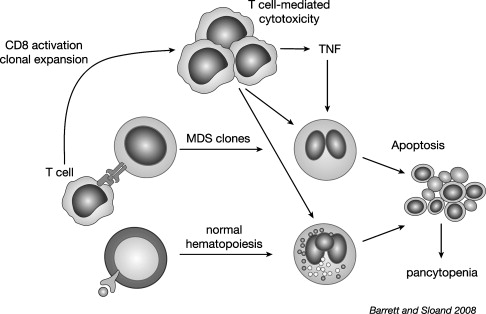
Early MDS is characterized by an increased apoptosis in marrow cells. It is not clear whether programmed cell death is related to alteration in genetic programming or is a result of an immune attack. Evidence that the ineffective hematopoiesis is immunologically mediated comes from experiments showing suppression of hematopoiesis by lymphocytes derived from patients with active disease, but not following successful treatment with ATG. Normally, quiescent lymphocyte populations contain a diverse distribution of thousands of individual clones within 23 T cell receptor (TCR) Vβ families. During an immune response, expansion of T cell clones specific for the corresponding antigen occurs. This expansion may be detected by flow cytometry using antibodies directed against the TCR Vβ subfamilies, or more specifically by spectratyping or CDR3 length analysis. Like patients with multiple sclerosis, those receiving vaccinations, and patients with infections, expanded Vβ subfamilies where they appear to be cytotoxic to hematopoietic cells present in some cases of MDS.
The mechanism for immunologically mediated marrow damage in MDS has been defined most clearly in patients presenting with trisomy 8 as a single karyotypic abnormality. The authors chose trisomy 8 to investigate, because such patients are likely to respond to IST. Additionally, the dysplastic clone can be identified readily by FISH. Initially, the authors showed that early apoptotic changes appeared to be restricted to the trisomy 8 clone, because flow cytometric-sorted Fas-positive, annexin-positive cells were enriched in, or entirely composed of trisomy 8 cells. T cells from expanded Vβ families selectively killed trisomy 8 cells in vitro, indicating that T cells could target trisomy 8 cells specifically. Consistent with this finding was the observation that depletion of cytotoxic T cells in BM cell improved growth of trisomy 8 colonies. Despite such specificity, it remained a possibility that normal BM cells could be killed as innocent bystanders by TNF and IFN-γ elaborated by activated T cells. Trisomy 8 cells survive despite showing signs of early apoptosis; DNA degradation did not develop, and cell death did not occur. The resistance of trisomy 8 cells to apoptotic cell death might be attributed to up-regulation of c-myc present on chromosome 8, and survivin, which may be responsible for resistance of trisomy 8 cells to T cell attack. Indeed, when these proteins are knocked down or inhibited, trisomy 8 cells generally die in vitro.
It may be hypothesized that CD8+ T cells recognize a neoantigen or an overexpressed self-antigen presented by major histocompatability locus (MHC) class 1 molecules on trisomy 8 cells. The authors searched for candidate antigens by examining microarray data on CD34 cells obtained from MDS patients with trisomy 8 and other cytogenetic abnormalities. Wilms tumor protein 1 (WT-1) was markedly overexpressed in trisomy 8 cells. WT-1 is a zinc finger transcription factor located on chromosome 11p13 and was implicated in the pathogenesis of Wilm tumor. WT1 is expressed at low levels in normal tissues, including kidney, ovary, testis, and spleen, but not in normal BM or peripheral blood (PB). It is overexpressed in various hematological malignancies including leukemia, MDS and paroxysmal nocturnal hemoglobinuria (PNH). Cytotoxic T cells recognize a number of epitopes from the WT1 protein.
In MDS, WT1 transcript levels increase with disease progression and correlate with the International Prognostic Scoring System (IPSS) stage. In the authors’ studies, WT1 m-RNA and protein were increased in many IPSS-intermediate 1 (int-1) MDS patients, but were highest in patients with trisomy 8. CD8+ T cells from patients who ultimately became IST responders generated TNF-α and IL-2 in response to WT1 peptide stimulation. CD8 cytotoxic T cells, recognizing WT1 126 peptide, also could be detected by tetramer analysis in HLA-A*0201-positive patients. These findings do not exclude the possibility that other antigens on MDS cells are recognized by the immune system, and that their identification will require further testing of peptide libraries. In this regard, PR1, a peptide derived from myeloid-restricted azurophil granule proteins, can provoke T cell responses in patients with MDS. In summary, therefore, the authors’ studies showed that MDS cells could overexpress tumor-specific antigens that are responsible for inducing autologous T cell cytotoxic T cell responses against the MDS lineage. In the case of trisomy 8, however, the MDS target cells are relatively resistant to such T cell-media apoptosis. Fig. 1 outlines the authors’ working model of T cell interactions with trisomy 8 cells that may be extrapolated to other MDS patient responsive to IST.
Patients with trisomy 8 have a high probability of responding to IST. Curiously, the authors found that selective patients may have normal bone marrow cytology despite the persistence of this karyotypic abnormally with trisomy 8 who recover their blood counts after IST to normal marrow morphology yet do not lose the trisomy 8 populations in the marrow. Indeed the percentage of trisomy 8 positive cells by FISH in blood and marrow can actually increase. Despite the increase in karyotypically abnormal cells, these patients remain stable for years and have shown a very low risk of progressing to acute leukemia. These observations reveal unique and unexpected consequences of the interaction of the immune system with MDS. First, in untreated patients, the survival of the trisomy 8 clone despite in the presence of the T cell response suggests that the MDS cells are adapted to survive T cell attack. Experimental data support this idea; trisomy 8 cells show signs of apoptotic induction with increased caspase and BCL-2 but appear to avoid destruction from T cell-mediated apoptosis by up-regulating survivin. This stalled apoptotic process results in “living dead” cells with dysplastic morphology. Second, the recovery of normal morphology in the trisomy 8 cells after IST strongly suggests that morphologic features of dysplasia at least in trisomy 8 disease may represent cellular responses induced by T cell attack. Lastly, and surprisingly, in the absence of T cell attack the expanded trisomy 8 clone can establish normal hematopoiesis, which remains stable for many years.
The place of immunosuppression in the treatment of MDS
Although BM transplantation is the only curative therapy in MDS, it is important to point out that about half of all deaths are related to cytopenias rather than leukemic progression. Any improvement in hematopoiesis thus may translate into improved survival for this disease. Following the considerable success of IST in SAA patients, this treatment was applied with some success first to hypoplastic MDS and then to normo- or hypercellular MDS. About one third of patients had become transfusion independent and enjoyed normal counts for years, but more commonly counts improved but did not revert to normal.
Cyclosporine-based treatments
Many early studies were performed using cyclosporine in low-risk MDS. Jonasova who treated 17 cytopenic patients with refractory anemia (RA) and variable marrow cellularity with cyclosporine (CsA) reported prolonged response in 82% of patients. All responders achieved transfusion independence. A multicenter study in Japan reported responses to CsA in 60% of 50 low-risk patients. Although CSA generally was well tolerated, renal failure occurred in a minority, necessitating stopping the drug. Others have reported no substantial responses to CsA and unacceptable toxicity. Together these findings indicate a potential role for CsA in the treatment of MDS with careful followup of renal function. It is important to note that responses occurred both in patients with hypocellular and hyper- and normo-cellular marrows.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree