Fig. 10.1
Schematic presentation of immune cells in the cancer microenvironment
In the setting of hepatocarcinogenesis, the most important predisposing factor is chronic HBV infection. The genome of HBV alters the expression profile of the host liver, resulting in the discordant proliferation of cells, and finally contributing to the onset of HCC. Recently, the paradigm is shifting and the role played by its immunopathology is being taken into account. Accumulating data indicate that chronic HBV infection induces long-lasting inflammation, which is considered as both innate and adaptive immunoresponses against the virus from the host [8]. The inflammation alone could induce the formation of HCC, even without evidence of productive viral infection [9]. Immune cells, cytokines, and growth factors in the inflammation lead to rapid turnover of liver cells and finally malignant transformation. Relevant participants have been extensively studied. Factors such as lymphotoxins (LT) are known to be related to the hepatocarcinogenesis [10]. Lymphotoxins and their receptors are up-regulated in hepatitis and HCC, and LT expression induces liver inflammation and HCC. Therefore, a causal link exists between LT overexpression to hepatitis and HCC. Sustained LT signaling represents a pathway involved in hepatitis-induced HCC. Toll-like receptors (TLR) are a broad family of proteins that recognize a broad spectrum of molecules shared among pathogens and referred to pattern recognition receptor. They play a key role in the pathogenesis of antimicrobe inflammation [11], supporting a role for chronic inflammation in hepatocarcinogenesis [12]. Besides, interleukin-6 (IL-6) is believed to contribute to the HCC formation. IL-6 binds to its specific receptor complex including the ligand-binding protein (gp80) and signal transduction protein (gp130) and regulates important signals such as JAK/STAT, ras/MAPK, and PI3K/AKT pathways [13]. Increased serum IL-6 level is reported in HCC patients compared to healthy controls, and positively related to larger tumor mass, more advanced stages, and more aggressiveness [14–16]. STAT3 is the most important mediator for the carcinogenesis activity of IL-6. STAT3 regulates the expression of important factors in the apoptosis, senescence, cell cycle, and angiogenesis [17]. Recently it was reported the STAT3 was constitutively activated in the HCC, but not in adjacent normal tissue [18]. The most direct link between inflammation and malignant transformation results from the activation of NF-κB pathway. It is well documented that NF-κB plays a critical role in inflammation, and recent lines of evidence show that it contributes to tumorigenesis, invasion, metastasis, and angiogenesis [19]. Overexpression of NF-κB is frequently observed in liver cancer tissues and HCC cell lines [20, 21]. NF-κB acts as a central link between hepatic injury, fibrosis, and HCC, and it may represent a target for the prevention or treatment of liver fibrosis and HCC.
Besides cytokines, immune cells involved in the inflammatory process contribute to carcinogenesis. Neutrophils are important in the inflammation response. They play paradoxical roles of both promoting cancer destruction and inducing the growth of cancer cells [22]. Cancer cells are known to produce chemokines, which act on CXCR2 receptors on neutrophils, and this ligand-receptor interaction leads to the release of VEGF-A by neutrophils that promotes tumor angiogenesis [22, 23]. Then, the neutrophils are recruited and induced to release VEGF or MMP, and both chemokines contribute to the invasion of endothelial cells and vessel formation. Then, angiogenesis follows and cancer progression is enhanced. The same principle applies to the HCC. Neutrophils in the HCC samples predict a shorter recurrence-free survival for HCC patients after liver resection [24, 25]. The number of neutrophils among the tumor margin strongly correlates with tumor angiogenesis and tumor progression [26].
Tumor-associated macrophage (TAM) is gaining focus in the immunopathology of cancer [27]. Growing evidence suggests that TAM promotes tumor growth and progression, instead of fighting against tumor as considered previously [28]. In a certain instance such as hypoxia, the TAM polarizes to a type 2 macrophage (M2)-like type [29]. M2-like TAM exerts a profound immunosuppressive effect by secreting and releasing cytokines such as CCL17, CCL22, or CCL24; then regulatory T (Treg) cells are recruited to the local milieu by these cytokines. In HCC, the intratumoral prevalence of regulatory T cells was correlated with the density of TAM [30]. TAM expresses programmed cell death (PD-1) ligand, which is considered as one of the most critical suppressive factors for immunity [31]. Additionally, M2-like TAM promotes angiogenesis through the production of VEGF or EGF [32]. In HCC, the TAM count was significantly correlated with microvessel density [33]. TAM was believed to be involved in the different prevalence of HCC between genders, where estrogen (E2) suppressed the macrophage alternative activation by inhibiting the JAK-STAT pathway [34]. TLR on the surface of HCC cancer cells recognizes and interacts with TAM, leading to recruitment of regulatory T cells in the microenvironment [35]. Recent studies suggested that the TAMs, together with regulatory T cells and hepatic stellate cells provided an immunosuppressive environment closely related to HCC recurrence [36].
Regulatory T cells (Tregs) are a unique subset of T cells with characteristic phenotype of CD4+, CD25+, and FOXP3+. Accumulating evidences suggest that Tregs play a prominent role in immune tolerance with the aim to prevent autoimmunity [37]. However, the shut-down of autoimmunity is at a price, and the tumor formation is facilitated at the same time. Recently, efforts have been put to elucidate the relationship between Tregs and hepatocarcinogenesis. Direct evidence comes from clinical observations. A high concentration of tumor-infiltrating Foxp3 Tregs in HCC is associated with high-grade and poorly differentiated tumors and signifies an unfavorable prognosis [38]. Tregs were reported to be associated with poor post-cryoablation prognosis in patients with hepatitis-B-virus-related HCC [39]. The number of Treg cells in HCC tissues could be used as a potential poor prognostic indicator for HCC patients after resection [40]. The prevalence of Treg cell was significantly higher in the peripheral blood and in tumor tissue compared with those in normal donors. The increased prevalence and expanded function of Treg cells in the tumor microenvironment of HCC were correlated to the cancer stage [41]. In order to provide the mechanistic explanation for the tumor-promoting activity of Tregs, one paper reported Tregs induced by HBV infection could suppress the antitumor immune response to HCC tumor antigen. This report therefore suggested that Tregs were involved in the immunopathogenesis of HCC [42]. Another report found that Tregs in the peripheral blood, pritumor, or intratumor sites of HCC patients exhibited different functional status. A higher prevalence and more suppressive phenotype suggested a critical role for intratumoral Tregs in the formation of multicellular immunosuppressive networks [43]. Yet another report examined the relation between γδ T cells and Tregs. The effector function of γδ T cells was substantially impaired in HCC, which is partially mediated by Treg cells [44]. Interestingly, not only classic CD25+FoxP3+ Tregs, but also noncanonical CD25−FoxP3− Tregs were found to have suppressive activities and were believed to take part in the liver cancer formation [45].
Compared to HCC, the immunopathology of cholangiocarcinoma and gallbladder cancer is less extensively studied. IgG4-related diseases consist of a broad spectrum of diseases with characteristics of marked infiltration by immunoglobulin G4 (IgG4)-positive plasma cells in affected organs [46]. Extrahepatic cholangiocarcinoma may be one of them. One study detected high prevalence (43 %) of IgG4 abundance in a total number of 54 cases with cholangiocarcinoma and gallbladder cancer [47]. The same study also suggested that cholangiocarcinoma cells could play the role of nonprofessional APCs and Foxp3+ regulatory cells. Another study examined the infiltration of IgG4-positive cells in 68 surgical specimens from patients with extrahepatic cholangiocarcinoma. Their results showed that ≥10 and ≥50 IgG4-positive cells per high-power field were found in 37 and 6 % of cases, respectively. In addition, the IgG4-positive cells showed a positive and negative correlation with FoxP3+ and CD8+ cells, respectively. Therefore, the study provided evidence that IgG4-positive cells in extrahepatic cholangiocarcinoma induced the evasion of immune surveillance associated with CD8+ cytotoxic T lymphocyte via the regulatory function of regulatory T cells [48]. B7-H1/PD-1 axis has been intensively studied as an important negative regulator for various cancers including HCC [49]. In the case of cholangiocarcinoma, although not so many, preliminary data showed that this axis played a role in its immunopathology. Totally, 31 intrahepatic cholangiocarcinoma specimens were examined by immunohistochemistry. Expression of B7-H1 and PD-1 was found to be up-regulated in cancer tissues compared with cancer adjacent tissues. Tumor-related B7-H1 expression was significantly correlated with both tumor differentiation and pTNM stage and was inversely correlated to CD8+ T cells [50].
The role played by TAM in cholangiocarcinoma was also explored. CD68+ and CD163+ macrophage infiltration was analyzed in paraffin-embedded tissue samples from 39 patients with intrahepatic cholangiocarcinoma where CD163 was used as a marker of M2 macrophages. The number of CD68+ and CD163+ macrophages was positively correlated with the numbers of vessels and regulatory T cells. Patients with high counts of CD163+ macrophages showed poor disease-free survival (p = 0.0426). The in vitro study suggested that STAT3 pathway was important for TAMs to facilitate tumor progression [51].
10.5 Current Therapies
The clinical management of HCC is the state of the art, especially given the insensitive nature of HCC to conventional chemotherapy or radiotherapy, with complicating underlying liver disease. A multidisciplinary medical team including experts of surgeons, pathologists, radiologists, and medical oncologists is required to provide care to patients with HCC efficiently and effectively.
Small, localized tumors are potentially curable. Patients with early-stage HCC (tumor size ≤5 cm, or ≤3 tumor with each ≤3 cm in size and without evidence of gross vasculature involvement) should be considered as potential candidates to receive curative partial hepatectomy [52, 53]. According to Milan criteria proposed in 1996, patients with small (tumor size ≤5 cm, or ≤3 tumor with each ≤3 cm in size and without evidence of gross vasculature involvement), unresectable HCC should be considered for liver transplantation [54]. Liver transplantation gives a 4-year overall survival and recurrence-free survival of 85 and 92 %, respectively.
For those who are not amenable to surgery, locoregional therapies should be considered. The latter modality contains two categories: ablation and embolization. For ablation, tumor control is achieved by exposure of the tumor to chemical substances (ethanol, acetic acid, etc.) or alteration of temperature (radiofrequency ablation, microwave ablation, etc.). Embolization refers to selective catheter-based infusion of particles to the arterials feeding the tumor, and this is achieved either by transarterial embolization, chemoembolization, or radioembolization. The expert panel from the American National Comprehensive Cancer Network of America recommends ablation alone for the small solitary tumor with tumor size ≤3 cm, and combination of ablation and embolization, embolization for lesions between 3 and 5 cm (www.nccn.org). With the rapid progress in radiotherapy techniques, adaptive external beam radiotherapy has been available for the treatment of HCC. The current radiotherapy such as stereotactic radiotherapy is capable of providing radiation beam to the tumor bed, while sparing the surrounding normal liver tissue. Radiotherapy has become an additional modality in the locoregional therapy of HCC. For the majority of patients with advanced disease, curative therapies are currently unavailable, and a palliative systemic therapy is preferred. Despite ample reports in the literature, the efficacy of cytotoxic chemotherapy is still in a state of debate. Sorafenib, an oral multi-kinase inhibitor, is the only agent for HCC which was evaluated in randomized control phase III trials [55, 56].
10.6 Progress in Immunotherapy
Although much effort has been devoted, the progress in therapy for HCC and biliary tract cancer remains limited and unfortunately, patients still have a disappointing prognosis. Only a minor proportion (approximately 20 %) of patients with HCC have the opportunity to get definite surgery, and others with advanced disease have to receive palliative therapy. With sorafenib, the only confirmed systemic agent, the overall survival in patients with advanced HCC improves by 2.3–2.8 months [55, 56]. The overall survival for patients with advanced-stage HCC is less than 1 year [57]. The prognosis of patients with advanced biliary tract cancer remains poor and the median survival time for those undergoing supportive care alone is short [58]. Given the disappointing efficacy of the currently available therapies, new therapeutic strategies are highly needed.
Cancer immunotherapy aims to treat cancer by eliciting anticancer immunity of the hosts to reject the cancer. Cancer immunotherapy is achieved by either cancer vaccine (active specific immunotherapy) or adoptive transfer of antibodies (antibody therapy) or immune cells (cell therapy). Serious doubts existed for many years as to whether the immune system is capable of eliminating human cancer. The effort was mostly in vain until the elucidation of mechanisms behind the immune recognition of tumor cells at the molecular level. T cells, through their T-cell receptors, specifically recognize tumor antigens that are processed and presented as small peptides in the groove of surface human leukocyte antigen molecules [59, 60]. Later came the milestone discovery when the first tumor-specific antigen MAGE-1 was discovered in the 1990s. Now, cancer immunotherapy is considered to specifically target tumor antigens and therefore is both efficient and safe. Since the report by Rosenberg and colleagues [61], our knowledge in the field of cancer immunotherapy has been rapidly increasing, and cancer immunotherapy will surely expand our armamentarium against cancer.
Although HCC was not considered “immunogenic,” lines of evidence indicates that the immune system plays a role in the formation and progression of the tumor (please refer to the immunopathology section). Also, preliminary observations suggest that the immune factor may help to suppress the tumor in vivo. For example, one preliminary study showed that lymphocyte infiltration in tumors was a favorite prognostic factor for patients with HCC [62]. Also, diminished frequency and impaired function of natural killer (NK) cells were described in HCC patients compared with healthy controls [63]. Not surprisingly, immunotherapy has been tested for HCC, and some of the protocols have been conducted in clinical trials. The following sections will discuss the immunotherapy at both preclinical and clinical levels.
10.6.1 Cancer Vaccines
Cancer vaccine aims at eliciting specific antitumor humoral and cellular immunity to eradicate the tumor or prevent their progression and spread. Promising progress has been achieved. The list of tumor vaccines includes protein/peptide vaccine, nucleic acid vaccine, anti-idiotype vaccine, recombinant virus vaccine, genetically modified tumor cell vaccine, and dendritic cell (DC) vaccine. Moreover, other novel types of tumor vaccines are emerging. They are mainly designed for specific antigens on the tumor cells or tumor-associated microenvironment. Accordingly, numerous studies have been performed with different cancer vaccines targeting different antigens on HCC.
The transmembrane 4 superfamily member 5 protein (TM4SF5) induces growth of HCC cells through the loss of contact inhibition and has been recognized as a potential antigen for HCC. Researchers formed a complex consisting of TM4SF5R2-3 epitope peptide and a special liposome complex as a vaccine [64]. Immunization with this vaccine in mice HCC models reveals both prophylactic and therapeutic effects. The results of this study suggested that this vaccine technology might be promising for future HCC patients with M4SF5-positive HCC.
Stem cells have received a great deal of attention for their clinical and therapeutic potential in cancer treatment. Researchers genetically modified stem cells to express cytosine deaminase (CD) and interferon-β and tested these stem cells as a vaccine to treat mice with HCC tumor burden. In the presence of the prodrug 5-fluorocytosine, the vaccine significantly inhibited the growth of the tumor mass [65].
The field of cancer immunotherapy has been strengthened by the discovery of vaccination with DC pulsed with tumor antigens is a potent strategy to elicit antitumor immunity (Fig. 10.2). DC is recognized as the most potent and efficient professional antigen-presenting cell identified so far, capable of activating both resting and naïve T cells. The ability to isolate and expand DC in vitro overcomes the previous obstacles in production. DC-based cancer vaccine has been considered as an attractive therapeutic approach. However, clinical trials often failed to confirm the efficacy of DC vaccine in patients, and this implied more dedicated modification needed to improve current strategy. Gao J et al. prepared a DC vaccine by pulsing DC with heat shock protein 70 from Mycobacterium tuberculosis with H22 tumor-peptide complexes and soluble CD40L [66]. Up-regulation of CD40, CD80, CD86, and HLA-DR expression was found, with higher level of T-helper type 1 cytokine secretion, such as IL-12p70, and resulted in the induction of H22-specific CTLs. Therapeutic administration of the vaccine significantly reduced progression of HCC tumors in mice.
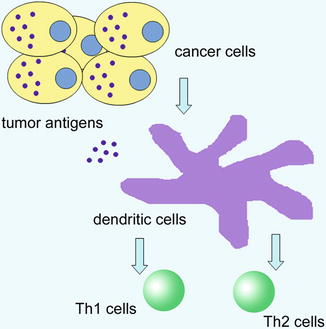

Fig. 10.2
Tumor antigen presented by DC and activation of T cells
For the design and development of cancer vaccine, the most important issue is the selection of a suitable tumor antigen as the therapeutic target. After decades of exploration, several tumor antigens have been proposed as potential targets for the development of cancer vaccines. These antigens are listed in Table 10.1. Among the antigens, Alpha-fetoprotein (AFP) may be one of the most well studied and promising. AFP is expressed during fetal development, but disappears shortly after birth. The relative unique distribution of AFP in HCC in an adult has long been harnessed for diagnosis of HCC. T cell epitopes were described in AFP. In a pioneer study, DC genetically modified to express AFP was capable of generating AFP-specific T cell response in peripheral blood mononuclear cells and transgenic mice [67]. Importantly, a 9-mer peptide (542–550) derived from AFP was identified as a potential A2.1-restricted peptide epitope. Later on, more epitopes from AFP were proved to induce AFP-specific T response in patients suffering from HCC [68]; these evidences strongly argue that AFP may serve as a therapeutic tumor antigen.
Table 10.1
Potential tumor antigens for immunotherapy of HCC
Tumor antigens | Expression frequency (%) | Detection methods |
|---|---|---|
AFP | ~80 | ELISPOT and tetramer |
GPC3 | ~70 | Cytotoxicity assay |
NY-ESO-1 | ~50 | ELISPOT |
MAGE-A | ~80 | Tetramer |
TERT | ~80 | ELISPOT |
Another potential antigen with promising prospects is Glypican-3 (GPC3). GPC3 belongs to a family of heparan sulfate proteoglycans, and it functions to bind growth factor and promote tumor growth [69]. GPC3 was found to be specifically expressed in HCC, and may serve as a diagnostic marker [70]. It indicated poor prognosis in HCC patients [71]. Later, the HLA-A24-restricted CTL epitopes were reported from GPC3 and indicated GPC3 was a possible tumor antigen for immunotherapy [72]. Based on these preclinical studies, a phase I clinical trial was conducted in Japan [73]. This registered trial (UMIN-CTR-000001395) recruited an overall of 33 patients with advanced HCC and escalating doses of GPC3 vaccines were administered. The GPC3 peptide vaccine was well tolerated. One patient achieved partial response and 19 patients had stable disease. Given the resistant nature of HCC and the small sample size in the phase I trial, results were promising. And shortly later, a phase II trial (UMIN-CTR-000002614) was performed. In this trial, the GPC3-derived peptide vaccine was used in the adjuvant setting. The primary endpoints were 1- and 2-year recurrence rate [74]. The authors are awaiting the results of this trial.
MUC-1 is another potential antigen which was proposed for cancer vaccine. In a study performed by Japanese researchers, a 100-mer MUC1 peptide consisting of the extracellular tandem repeat domain and incomplete Freund’s adjuvant were administered to several patients including three bile duct cancer patients [75]. The study showed that the vaccine was safe and well tolerated; however, no other conclusion was drawn due to the small sample of the phase I trial. However, no sequelae study was reported for this vaccine.
DC vaccines were tested in clinical trials. In one recently published report, DC vaccine prepared by pulsing DC with a liver cancer cell line lysate was administered to 15 patients with advanced HCC [76]. The vaccine achieved two cases of partial response and nine cases of stable disease. Both cellular and humoral immunity were elevated after DC vaccine inoculation. Another similar study was performed in China. In this trial, DC was pulsed with synthesized α1,3-galactosyl epitope modified tumor cells, and totally nine patients received the DC vaccine [77]. The vaccine significantly prolonged the survival of patients as compared with the controls (17.1 ± 2.01 months vs. 10.1 ± 4.5 months, P = 0.00121). Elevated level of interferon was detected after DC vaccine inoculation. These pilot studies indicated the possibility of translating the success of DC vaccine in preclinical studies to human subjects.
10.6.2 Cell Therapy
Adoptive cell therapy involves the transfer of immune cells with antitumor reactivity. Adoptive cell therapy aims at tumor elimination through direct or indirect effects of repairing or enhancing the immune function. Early studies involved the transfer of lymphokine-activated killer (LAK) cells with nonspecific ability to recognize and lyse tumor cells in vitro to cancer patients [78]. The use of T cells for adoptive therapy may be more attractive, because of their ability to specifically target tumor cells, besides long clonal life span. This strategy of adoptive cell therapy achieved some success in pilot studies where patients with melanoma or renal carcinoma were treated [79]. Further trials concentrated on isolation, propagation, and activation of highly active and avid tumor-specific T cell clones and adoptive transfer for cancer patients. T cell expression of inhibitory proteins can be a critical component for the regulation of immunopathology, but it may also limit T cell responses to malignancies. In a recent report, researchers abrogated the expression of the Src homology region 2 domain-containing phosphatase-1 (SHP-1) in tumor-reactive CD8+ T cells [80]. Following in vivo transfer, the SHP-1(−/−) effector T cells exhibited enhanced short-term accumulation, followed by greater contraction, and ultimately formed similar numbers of long-lived, functional memory cells. The increased therapeutic effectiveness of SHP-1(−/−) effector cells was also observed in recipients that expressed the tumor Ag as a self-antigen in the liver. In another report, it was attempted to improve the efficacy of cytokine activated cells (CAK) in HCC by combining them with the chemotherapy agent gemcitabine [81]. In this study, gemitabine treatment led to increased expression of MHC class I chain-related A and B on the surface of HepG2 HCC tumor cells, both of which were recognized as ligands for activating receptors on NK cells. Pretreatment with gemcitabine and CAK cells induced greater cytotoxicity than either treatment alone.
An important randomized control study performed in Japan strongly argued for adoptive immunotherapy for postsurgical HCC patients [82]. In this trial, a total 150 patients who underwent curative surgery for HCC were enrolled. Half of them (n = 76) were assigned to receive adoptive transfer of IL-12 and anti-CD-3 antibody-activated lymphocytes, and the other patients received no adjuvant therapy. Adoptive immunotherapy decreased the frequency of recurrence by 18 % compared to the control group. The immunotherapy group had significantly longer recurrence-free survival (p = 0.01) and disease-specific survival (p = 0.04) than the control group. In support of this study, a systematic review was recently published where the authors evaluated the efficacy of adjuvant adoptive immunotherapy for postsurgical HCC patients [83] in which four randomized control trials with 423 patients were eligible. All trials reported significantly improved disease-free survival rate or reduced recurrence rate after treating with adjuvant adoptive transfer of cells (p < 0.05). This study adds to the evidence that postoperative HCC patients treated with adjuvant cell therapy show improvement in disease-free survival rate or recurrence rate. In another study, CAK cell therapy was combined with microwave ablation therapy for HCC [84]. In a phase I study, adoptive transfer of DC, CAK, and CTL cells was conducted together with microwave ablation. The aim of this study was to observe the viral load before and after the combination therapy. After therapy, the viral load was significantly lower, the number of Tregs decreased, and effector T cells increased. However, this study did not report the clinical efficacy of the combinatory regimen.
10.6.3 Antibody Therapy
An important aspect of immunotherapy is antibody therapy. Antibody therapy is in the rapid boosting stage these days, and the list of novel antibody therapies is rapidly increasing. Among the antibodies available for cancer immunotherapy, bevacizumab is recognized as one of the most important for its unique inhibitory effects on tumor vasculature but not on tumor cells. Tumor angiogenesis has been proved to be critical for a panel of tumors, including HCC [6]. HCC is among the highly angiogenic cancers; disorganized and tortuous vasculature was reported in HCC [85]. The poor vasculature led to insufficient infiltration of nutrition, oxygen, therapeutic drugs, as well as lack of immune active cells. The concept of anti-angiogenic therapy was tested in HCC. In a preliminary study, a segment of oligodeoxynucleotides with cytosine-guanine-rich (CpG) motifs was used according to a classical vaccination protocol [86]. This kind of vaccine led to vasculature remodeling, rendered tumor permissive for infiltration of immune cells, and demonstrated antitumor efficacy. Given that the small molecular anti-angiogenic agent, sorafenib, is confirmed to possess antitumor efficacy for HCC, then how about bevacizumab? Several studies tried the bevacizumab for HCC patients. In a recently published phase II trial, 43 patients received bevacizumab [87]. Of the patients, six (14 %) achieved a partial response. The disease control rate at the time point of 16 week was 42 % (95 % confidence interval, 27–57 %). In addition, circulating endothelial cells were found to be associated with response, while interleukin-8 and -6 were negatively related to the therapeutic efficacy. A systematic review analyzed the efficacy of bevacizumab for HCC, although all trials eligible were phase II trials [88]. Eight trials involving 300 patients were included. The results favored the use of becizumab either alone or in combination with other agents. Phase III trials are warranted to comprehensively examine the efficacy and safety of bevacizumab for treatment of advanced HCC.
Recently, the immune regulatory mechanism becomes the focus of cancer immunotherapy. Tumor harnesses numerous regulatory pathways to evade immune surveillance, but these negative regulatory mechanisms provide additional therapeutic targets. The proof-of-concept evidence comes from the anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) antibody. CTLA-4 is a cell surface molecule expressed almost exclusively on CD4+ and CD8+ T cells, and is important for the maintenance of T cell homeostasis [89]. CTLA4 on the surface of T cells binds to CD80 and CD86 on antigen-presenting cells, and transmits an inhibitory signal to T cells. The CTLA-4 antibody ipilimumab is proved to be capable of inducing objective response in variant tumors, especially melanoma [90]. In one study, the effects of CTLA-4 blocking antibody were tested in vitro [91]. Along with a panel of tumor-associated antigen peptide vaccine, ipilimumab resulted in unmasking of immune responses by changing cytokine or chemokine profiles in peripheral blood mononuclear cells. These results suggested that ipilimumab had a role in the immunotherapy of HCC, but further studies are needed to confirm this hypothesis.
The antibody induces the glomerization of series of complements. The complex formed by complements has a direct tumoricidal effect by attacking the cell membrane or releasing a signal for other effector cells (opsonizing). Fc fragment also binds specific receptors on the surface of effector cells like NK cells or T cells, and this antibody-dependent cytotoxicity (ADCC) effect plays an important role in the antitumor effect of the antibody. Equally promising is the use of conjugate antibody (Fig. 10.3). Conjugated antibody comprises two parts: the conjugates and the antibody. The antibody itself does not possess antitumor activity, but rather performs to carry the conjugate (“magic bullet”) such as isotope (for instance, Tuxuetan), toxin, or chemotherapy agent to the tumor site.
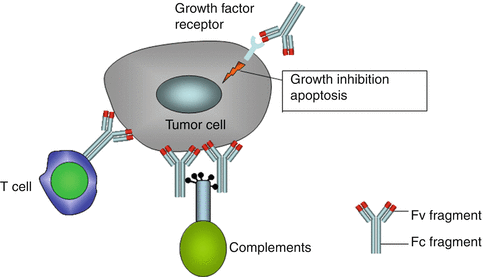

Fig. 10.3
Mechanisms of antitumor efficacy of antibodies
Because of its great therapeutic and economical potential, efforts have been devoted to the research and design of novel antibody agents, and a radio-labeled antibody-131I labeled Metuximab injection (Licartin) for treatment of HCC has been developed and become commercially available in China.
In a pilot study, the researcher recruited 24 HCC patients and randomly divided them into three groups to receive 18.5, 27.75, and 37 MBq/kg of Licartin per kilogram of body weight, respectively. Licartin was injected by hepatic artery intubation. The positive imaging result of monoclonal antibody (mAb) scanning in 24 patients showed that Licartin was apparently accumulated more in hepatoma. These data supported that 131I-labeled Metuximab could deliver relatively selective radiation to tumor tissues [92]. They also carried clinical trials to demonstrate that Licartin was safe and active for HCC patients. In the phase I trial, 28 patients were randomly assigned to receive the injection in 9.25-, 18.5-, 27.75-, or 37-MBq/kg doses by hepatic artery infusion. In a multicenter phase II trial, 106 patients received the injection (27.75 MBq/kg) on Day 1 of a 28-day cycle. Response rate and survival rate were the endpoints. No life-threatening toxic effects were found. The safe dosage was 27.75 MBq/kg. The blood clearance fitted a biphasic model, and its half-life was 90.56–63.93 h. In the phase II trial, the injection was found to be targeted and concentrated to tumor tissues. Of the 73 patients completing two cycles, 6 (8.22 %) had a partial response, 14 (19.18 %) minor response, and 43 (58.90 %) had stable disease (SD). The 21-month survival rate was 44.54 %. The survival rate of progression-free patients was significantly higher than that of patients with progressive disease after either one or two cycles (p < 0.0001 or p = 0.0019) [93].
The antibody Licartin was also shown to be effective in preventing hepatoma recurrence after liver transplantation in a randomized controlled trial. This trial was to assess the post-orthotopic liver transplantation (OLT) anti-recurrence efficacy of Licartin in advanced HCC patients. Sixty post-OLT patients with HCC, who were at tumor stage three-fourth and outside the Milan criteria before OLT, were randomized into two groups. Three weeks after OLT, the treatment group received 15.4 MBq/kg of Licartin, while the control group received placebo intravenously for three times with 28-day intervals. At 1-year follow-up, the recurrence rate significantly decreased by 30.4 % (p = 0.0174) and the survival rate increased by 20.6 % (p = 0.0289) in the treatment group, compared with those in the control group. For the control group versus the treatment group, the hazard ratio for recurrence was 3.60 (95 % confidence interval [CI], 1.50–8.60) and that for death was 3.87 (95 % CI, 1.23–12.21). Licartin treatment also resulted in an earlier decreased AFP level and a longer time of normal AFP level than placebo (p = 0.0016). No Licartin-related toxic effects were observed. The authors concluded that Licartin is a promising drug for preventing post-OLT tumor recurrence in advanced HCC patients excluded by the currently strict criteria for OLT [94]. However, human anti-mouse antibody (HAMA) response in some patients after administration limited its clinical use of Licartin. Therefore, attempts were made to develop a more efficient antibody fragment with less immunogenicity. To reduce the immunogenicity of murine antibody, they attempted to humanize HAb18 by variable domain resurfacing based on the three-dimensional structure of Fv fragment. They fabricated a humanized version of HAb18scFv, HAb18-huscFv, to the human IgG1Fc fragment to form (HAb18-huscFv)(2)-Fc. The reactivity of (HAb18-huscFv)(2)-Fc to the serum of patients with HAMA response was decreased, while its specificity and similar binding activity remained intact [95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



