Fig. 7.1
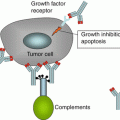
The figure demonstrates the immune environment of HRS cells in HL, as well as the antibodies located on the cell surface which are considered potential targets for immunotherapy
Naked Abs are dependent on immune effector cell activation for implying their therapeutic effects. In addition, effector immune cells are targeted by T-regulatory cells. Thus, an immunosuppressive environment is induced, explaining the low therapeutic efficacy of naked mAbs in clearance of HRS cells [22]. All the abovementioned mechanisms provide explanations to the low efficacy of several therapeutic Abs employed in HL involving effector cell-dependent antitumor activities [5]. The low mitotic index (0.5 %), due to mitotic defects and high degrees of apoptosis [23], contributes to the low efficacy of Abs directed toward tumor Ags, targeting as low as 0.5–5 % of the tumor mass cell population [24].
HL tumors, classified as a liquid tumor, possess a solid tumorlike appearance and composition. Antibodies and antibody-drug conjugates are poorly effective on solid tumors, and only 0.001–0.01 % of the injected antibody permeates the tumor [25]. Therefore, the efficacy of immunotherapy in HL is mitigated due to the solid tumorlike composition, as well as the low frequency of HRS cells [5].
Tumor necrosis factor receptor (TNFR) superfamily includes CD30, CD40, Fas (CD95), and OX40 (CD134), just a few to point to [26]. CD30-ligand binding or cross-linking by immobilized Abs, ultimately provokes biological signals including cell proliferation and apoptosis [5]. CD30-CD30 ligand interactions on the surface of HRS cells are recognized in the pathogenesis of HL [27]. Due to the considerable difference between the ability of anti-CD30 Abs in the interference with NF-kB signaling pathway, their therapeutic efficacy is variable [5]. MAP kinases and NF-kB are the hallmark events regulated by CD30 signaling pathway [28, 29], leading to cell proliferation and survival, in addition to induction of antiproliferative responses and cell death [30]. Activation of NF-kB leads to the expression of antiapoptotic genes such as cFLIP [31], XIAP [32], and Bcl XL [33], hence posing an additional level of complexity to the treatment of HL [34]. Therefore, inhibition of the NF-kB signaling pathway by therapeutic Abs seems a beneficial interference [34].
The active, phosphorylated form of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MAPK, EPK; p44/42) is observed in cultured and primary HRS cells. Furthermore, inhibition of the upstream kinase EPK has been shown to be associated with decreased growth of HL cell lines [35]. Hetero- and homodimers of Jun, Fos, and other members of the activating transcription factor (ATF) family, c-Jun, and JunB are found to be expressed excessively in HL and ALCL, but not in other subtypes [36]. On the other hand, inhibitors of the phosphatidylinositol kinase/AKT pathway are recognized as potential inducers of apoptosis in HRS cells [37]. The certain morphological feature of HL tumor, along with alterations in major signaling pathways, justifies the lack of the efficacy of conventional antibody-based immunotherapeutic treatments and highlights the inevitable need to novel therapeutic strategies [5]. Moreover, the expression of delayed hypersensitivity is found to be impaired in these patients.
T cells play a critical role in the HL cell microenvironment, as they compromise the majority of infiltrating cells. HRS cells are directly surrounded by CD4+ T cells, which do not have a Gemcitabine and carboplatin (GC) Th-cell phenotype, and comprise a mixed population of Th cells with Th2 phenotype and regulatory T cells (T reg) [38, 39]. MHC II, a crucial mediator of the interaction between T cells and B cells, is downregulated in 40 % of HRS cells [40]. HRS cells attract chemokine receptor CCR4-Th and Treg cells via the secretion of large amounts of the CCL17 chemokine [38]. Overall, studies have indicated that the HRS cell/T-cell interaction plays an important role in the pathogenesis of HRS cells. Various factors contribute to the interaction between CD4+ T cells with HRS cells, including the production of IL-13, an HRS cell growth factor produced by T cells [41], and stimulation of HRS cells through interaction between CD28 (expressed on T-cell surface), CD80 and CD86 (expressed by HRS cells), and CD40 on HRS. Tumor-infiltrating Treg cells preserve HRS cells from the attack of cytotoxic T and NK cells, via the production of IL-10 and the expression of T lymphocyte-associated protein 4 (CTLA4) [10]. Therefore, Treg cells play a suppressive role in the HL microenvironment. HRS cells are recognized to draw Treg cells into the lymphoma microenvironment; in addition, they play a direct role in the differentiation of CD4+ T cells to Treg cells [42]. HRS cells directly inhibit cytotoxic T lymphocyte and NK cells in cHL, through production of IL-10, TGF, gelatin-1, tissue inhibitor of metalloproteinase 1 (TIMP1), and prostaglandin E2 (PGE2) [43, 44]. On the other hand, ligands PD1 and 2, which inhibit PD1 T cells and express CD95 ligand, are also expressed on HRS cells, resulting in the apoptosis of CD95-activated CD8 and Th1 T cells [43, 45]. Figure 7.1 well depicts the microenvironment of an HRS cell.
As explained by the immunopathology, the main focus of immunotherapy in HL is directed toward activation of T-cell responsiveness, particularly around HRS cells. Reactivating an antitumor immune response by pinpointing cytokines capable of reversing the polarized immune response has been advocated in this regard; in addition, antibody-targeted cytokines which accumulate in the lymphoma lesion have been found to be more efficacious compared to unmodified cytokines. These findings shed light on the importance of the application of antibody-cytokine fusion proteins for targeting cytokines toward HRS tumor cells, combined with the advantages of local reactivation of immune responses [46]. HRS cells express multiple cell surface Abs, among which a variety are considered potent targets for immunotherapy [47].
CD1d and NK cells also play a pivotal role in the pathogenesis of HL. CD1d, normally expressed on hematopoietic cells of myelomonocytic and B-cell lineages, is a marker for malignancies originating from the corresponding tissues.
B-cell malignancies have also been found to display CD1d. Studies on murine models have demonstrated the expression of CD1d on many leukemia and lymphoma cell lines. Moreover, NKTs have exhibited a protective role in the A20 murine B-cell lymphoma model [48], which is correlated to the level of CD1d expression on lymphoma cells, and was lost in NKT-deficient mice. Studies on human lymphomas have revealed that CD1d is expressed on the surface HRS cells in half of the cHL cases [49]. Notably, NKTs were present at high frequencies in primary cHL tumors and reactive lymph nodes irrespective of CD1d expression on tumor cells. However, the functional role of tumor-infiltrating NKTs in cHL biology and disease outcome is yet to be determined. It is postulated that NKTs may co-localize with CD1d-positive tumor-associated monocytes/macrophages (TAMs) in the microenvironment of CD1d-negative tumors [50]. In addition, the increased number of TAMs is significantly correlated to decreased survival rates in patients with cHL [51]. Targeting both HRS cells and TAMs for immunotherapy with NKTs and/or their ligands seems a promising approach [52]. The strongest known risk factor for the development of lymphoma is immunosuppression, predominantly NK cell dysfunction. NK cells are critical effectors in tumor immunology and were usually regarded as effector cells of innate immunity. However, more recently, it has been shown that they attribute to both innate and adaptive immunity, playing a regulatory role in shaping antigen-specific T- and B-cell responses [53]. NK cell activity is significantly impaired in HL compared to controls, irrespective of histological type and clinical stage. Notably, the most profound NK cell dysfunction, present and persistent in HL, is associated with increased LDH release activity from peripheral blood mononuclear cells. NK cell function is greatly impaired in HL; in addition, impaired NK cell activity is associated with increased spontaneous release activity of LDH from patients’ PBL, which is indicative of cell membrane damage, followed by the release of cytotoxic proteins, and eventually impaired NK cell activity [4].
7.3 General Concepts of Monoclonal Antibodies
7.3.1 The Structure of Monoclonal Antibodies
Antibodies used in immunotherapy target the Ags specifically present on tumor cells. The mAb technology was developed by Kochler and Milstein [54]. Monoclonal antibodies consist of a Fab and an Fc region, resulting in a Y-shape structure. The Fab fragment contains the complementary-determining region (CDR), which defines the specificity of a mAb toward the Ags, whereas the Fc fragment, an isotype IgG, is responsible for the Ab’s mechanism of action and interacts with cells expressing Fcg receptors (FcgR) on immune cells including natural killer (NK) cells, macrophages, and neutrophils [55]. FcgR stimulation leads to activation of the ADCC pathway and results in cytotoxic events. In addition, complements are fixated by Fc fragments, ensuing the activation of the Complement-dependent cytotoxicity (CDC). Direct intracellular signal, resulting in an antiproliferative effect and apoptosis, is proposed as an alternative mechanism of action, which is activated by direct binding of the mAb to its Ag [55].
7.3.2 Choosing the Optimal Antibody
The exquisite specificity of Abs renders them ideal targets for immunotherapy in malignancies including HL and NHL. Selective cancer immunotherapy was first proposed by Paul Ehrlich [56]. Successful immunotherapeutic approaches rely on appropriate target Ag selection. In selecting the appropriate antibody-based therapies, certain conditions should be taken into account, including (1) selectivity of the antigen expressed on the target cell and its sufficient expression; (2) maintenance of the antigen on the cell membrane; (3) no internalization after mAb binding, and (4) its ability in initiating a cytotoxic effect upon binding to its target.
IgG1 is the most widely used human therapeutic Ab isotype [57]. It has the appeal of prolonged half-life compared to other human IgGs [58]. In addition, it exhibits significantly greater specificity and affinity to activation and inhibition of FcgR. Finally, it results in greater ADCC induction [59].
Monoclonal antibodies are named based on their origin; their general nomenclature is as follows: The addition of -omab, -amab, and -emab to the generic name of the Ab each implies murine, rat, and hamster origins, respectively, whereas adding -imab, -ximab, -zamab, and -umab each indicates primate, chimeric, humanized, and human origins, respectively [55].
Herein, Ags targeted in immunotherapy of HL, along with the advances made in immunotherapeutic approaches, have been discussed.
7.4 CD30
CD30, a member of TNF receptor superfamily, is expressed on active B and T lymphocytes and NK cells. In addition, it is considered a diagnostic immunomarker for classical HL (cHL), as it contributes to the proinflammatory tumor microenvironment [60]. Through interaction with the TNF receptor-associated factor 2 and 5 (TRAF 2 and 5), activation of NF-kB is pursued. Thereafter, apoptosis and proliferative potential of autoreactive T cells are regulated [55]. CD30 is expressed on the cell surface of HRS cells [61]. Due to the paucity of CD30 expression on nonimmune system cells, it is considered a potential target for immunotherapeutic approaches for HL [62]. CD30-CD30 ligand interaction on the surface of HRS cells is recognized in the pathobiology of HL (Fig. 7.1) [27]. Under normal conditions, CD30 is solely expressed on activated NK cells, monocytes, eosinophils, and a small proportion of large lymphoid cells in sections of lymph nodes, tonsil, thymus, and endometrial cells. Serum CD30 level has been found to correlate with the prognosis of HL [63]. Due to the paucity of expression in nonneoplastic cells outside the immune system, it is regarded as an exquisite candidate for mAb therapy [64]. Nonetheless, the paucity of malignant CD30+ (HRS) cells poses an additional level of complexity to CD30-targeted therapy [65]. The tumor mass in HL is defined as CD30+ malignant cells surrounded by massive infiltrations of immune effector cells, which apparently have failed to clear the cell mass in the involved lymph nodes [9]. Due to considerable differences between the ability of anti-CD30 Abs in interfering with NF-kB signaling pathway, their therapeutic efficacy is variable [5]. MAP kinases and NF-kB are the hallmark events regulated by CD30 signaling pathway [28, 29], leading to cell proliferation and survival, as well as induction of antiproliferative responses and cell death [30]. Activation of NF-kB leads to the expression of antiapoptotic genes such as cFLIP [31], XIAP [32], and Bcl XL [33], hence posing an additional level of complexity to the treatment of HL [34].
Various mAbs targeting CD30 have been evaluated in preclinical tumor models (Table 7.1) [75–78]. CD30 monoclonal Abs may trigger cell death directly or indirectly through ADCC or CDC or via Ag-dependent cellular phagocytosis (ADCP). Gerber et al. [5] described different physiobiochemical properties of various CD30 mAbs, leading to the induction of unique set of pharmacodynamic responses, discrepancy in epitope recognition, binding affinities, and effector cell activation characteristics, only a few outcomes to point to. Accordingly, various anti-CD30 Abs including AC10, Ki-1, 5F11, M67, and Ber-H2 demonstrated distinct domain recognition during cross-blocking competition studies [75, 79]. Moreover, only 5F11 and AC10 are found to interfere with human HL cell line growth in culture [80–82]. More precise descriptions on each anti-CD30 Ab are provided below.
Table 7.1
CD30-directed immunotherapy in HL
Drug | Study type | N | CR + PR(%) | Reference |
|---|---|---|---|---|
Murine anti-CD30-saporin conjugate (Ber-H2/SO6) | Pilot | 4 | 75 | [63] |
Murine anti-CD16/CD30 | Phase I/II | 15 | 13 | [66] |
Murine anti-CD16/CD30 combined with IL-2, GM-CSF | Pilot | 16 | 25 | [67] |
Anti-CD64/CD30 | Phase I | 10 | 40 | [68] |
Murine anti-CD30-ricin-A conjugate (Ki-4.dgA) | Phase I | 17 | 7 | [69] |
Murine anti-CD30-131 iodine-conjugate | Phase I | 22 | 27 | [70] |
Chimerized anti-CD30 mAb (cAC10, SGN-30) | Phase I | 13 | 15 | [5] |
cAC10, SGN-30 | Phase II | 35 | 0 | [71] |
Humanized anti-CD30-mAb, (MDX-060) | Phase I/II | 72 | 8 | [72] |
cAC10-auristatin conjugate (cAC10-vcMMAE, SGN-35) | Phase I | 39 | 45 | [73] |
Humanized, effector cell-enhanced anti-CD30 mAb(parental MDC-060, MDX-1401) | Phase II | 72 | 8 | [74] |
7.4.1 CD30 Monoclonal Antibodies
7.4.1.1 MDX-060 (5F11)
5F11, a hybridoma-derived antihuman CD30 IgG1 Ab, is a well-established inhibitor of the growth of cells expressing CD30, in vitro, acting via the induction of growth inhibitory cell signaling and ADCC pathways and eventually leading to efficient cell apoptosis. It is generated in human transgenic mice, and its optimal anti-HL effect was established in disseminated and solid murine models with human HL [75]. Borchmann et al. demonstrated that treating mice implanted with solid or disseminated HD tumor cells (L540cy) leads to reduced tumor volume and increased survival [75]. Moreover, its additive effect was observed in combination with conventional cytotoxic drugs, particularly with gemcitabine and etoposide, in vitro, leading to increased sensitivity to chemotherapy [78]. During a phase I clinical study on patients with refractory HL, a dose up to 15 mg/kg was proved to be safe [72]. However, further studies regarding combination therapy with cytotoxic drugs seem mandatory.
7.4.1.2 MDX-1401
MDX-1401, the non-fucosylated version of MDX-060, is superior to MDX-060 due to increased ADCC activity. Hence, lower doses are required to achieve the same ADCC activity [74].
7.4.1.3 Chimeric-AC10
Chimeric-AC10, which is similar to human IgG1 subclass in structure, promotes growth arrest and DNA fragmentation of CD30 positive tumor cells, thereby inducing its antitumor effects [83]. In vitro experiments revealed that ADCP plays a pivotal role in the antitumor activity of chimeric-AC10 [22]. Moreover, chimeric-AC10 was found to boost the antitumor activity of bleomycin in HL xenografts [84]. The domain on CD30 recognized by AC10 differs from the domain bonded by Ki-11, 5F11, or Ber-H2 [75, 79].
7.4.1.4 SGN-30
SGN-30, a chimeric IgG1 mAb derived from the murine AC10 anti-CD30 mAb, has demonstrated an antiproliferative effect in vitro and a potent anti-HL effect in xenografts [83]. Macrophages play a critical role in the activity of SCN-30, proven by the abolished effect of SGN-30 in the absence of macrophages in experimental studies [83]. It has proved as a safe and well-tolerated mAb, yielding adequate response in patients with HL during phase I and phase II [85, 86]. Remarkably, HL cell lines treated with SGN-30 were sensitized to conventional cytotoxic drugs, including bleomycin and etoposide [84]. However, combination of SGN-30 with conventional chemotherapy in a phase III study led to development of pneumonitis in a considerable proportion of patients, posing a potential limitation to its administration combined with chemotherapy. Particularly, FcgRIIIa-158 V/F polymorphism was associated with an increased risk [87].
7.4.2 CD30 mAb-Drug Conjugates
The combination of chemotherapy with immunotherapy has revealed enhanced efficacy and has been clearly effective in extending survival [88]. Some limitations to effective immunotherapy are overcome by applying antibody-drug conjugates (ADCs). Since ADCs apply their therapeutic effect independent of inflammatory cells, immune evasion mechanisms are not involved. Hence, lower exposure levels are required compared to naked Abs. In addition, restricted access of macromolecules to tumor cells has been overcome by combining ADCs to cytoreductive chemotherapeutic agents. Interestingly, immense increase in the amount of local active drug in malignant cells is observed by applying ADCs, which could compensate for the small fraction of malignant HRS cells in HL tumor mass [5]. ADCs have the appeal of being administered over a prolonged time with no treatment holidays, as their toxicity profile has been reduced compared to cytotoxic drugs [5]. In the following CD30 mAb-drug conjugates, studies in HL are discussed.
7.4.2.1 Brentuximab Vedotin
Brentuximab vedotin (SNG-35, ADCETRIS™, Seattle Genetics) was approved by the FDA for the treatment of cHL and ALCL in 2011. Brentuximab, a CD30 mAb, comprises the Ab section, whereas a microtubule-disrupting agent, monomethyl auristatin E (MMAE, three to five units), comprises the drug section. Once brentuximab vedotin binds to the CD30 receptor on the cell surface, CD30-drug complex is internalized, and cytotoxic components are released as a consequence of proteolytic cleavage in the lysosome. Remarkably, few brentuximab vedotin molecules are sufficient to achieve clinical efficacy, making it favorable for HLs with low CD30 expression [89]. Moreover, brentuximab vedotin leads to decreased levels of chemokines and cytokines (TARC), which resolve the inflammatory infiltrate and disrupt the microenvironment, and in turn, facilitate the antitumor immune response [90]. It has been suggested that combining brentuximab vedotin with chemotherapeutic regimens yields promising results.
7.5 CD20
CD20, a protein of 297 amino acids with four transmembrane regions, is exclusively expressed on the lymphocytic and histiocytic cells of nodular lymphocyte predominant HL (NLPHL). The expression of CD20 in this subtype is recognized as a diagnostic hallmark, distinguishing it from cHL [91, 92]. Some studies have investigated its efficacy in NLPHL, which would be briefly discussed in the following.
7.5.1 Rituximab
Wirth et al. have described beneficial outcome with the administration of rituximab in patients with relapsed stage IA NLPHL. The GHSG and Stanford trials conducted on relapsed patients observed a 93 and 100 % ORR, respectively. Four weekly administrations of rituximab (375 mg/m2) as a frontline therapy in the GHSG study on 28 patients yielded an ORR of 100 % and an 86 % CR. As concluded in their study, rituximab-based combination treatment holds promising potential as the frontline treatment [93]. Nineteen newly diagnosed NLPHL patients recruited in the study by the Stanford group manifested a 100 % ORR and a 63 % CR [93]. The study was extended to 2 years with repeated four weekly infusions every 6 months which improved CR to 88 %. In the same line, limited rituximab therapy in NLPHL patients in the GHSG study resulted in 94 % ORR and 53 % CR [94]. Moreover, a phase one half trial has been conducted on relapsed NLPHL patients using tositumomab, a first-generation type II CD20 mAb, and 131I-tositumomab. Patients received 450 mg single dose, leading to CR in all patients. Cytopenia was regarded as the most common adverse event [95].
Nonetheless, the application of CD20 mAbs is restricted in HL and is mainly specific to NHL. Accordingly, further description on the application of CD20 mAbs is provided in the next chapter.
7.6 CD40
CD40, a member of the tumor necrosis factor receptor family, is highly expressed on neoplastic B cells. Stimulation of CD40 leads to immunoglobulin isotype switching and activation of B cells, eventually resulting in enhanced proliferation and survival. Remarkably, it is recognized as an independent risk factor for some hematological malignancies [96]. Most studies in the literature are conducted on NHL patients, with only a handful of data available on its effect in HL.
7.6.1 Lucatumumab (HCD122)
Lucatumumab (HCD122), a CD40-targeted mAb, was studied in a phase IA/II study in order to determine its maximum tolerated dose (MTD) and activity. Escalating doses of lucatumumab administered intravenously once weekly for 4 weeks of an 8-week cycle were administered in 37 patients with relapsed HL. Finally a MTD of 4 mg/kg and modest activity were manifested, necessitating further investigations to establish its benefits in the clinical setting [97].
7.7 CD80
CD80 (B7-1) is a co-stimulatory molecule aberrantly expressed on HRS. Various anti-CD80 mAbs have been developed, most of which have been studied in NHL, whereas only a few studies have addressed their implication in HL.
7.7.1 Galiximab (IDEC-114)
Galiximab, a chimeric mAb against CD80, has manifested favorable toxicity profile in NHL, while its activity in HL is rarely studied. Just recently, the Cancer and Leukemia Group B (CALGB) 50602 (Alliance) investigated its efficacy in highly refractory HL patients who had previously received a median of three prior regimens, 83 % failing after prior stem cell transplant. Disappointingly, an ORR as low as 10.3 % and only 1.6 months PFS was achieved, indicating its limited activity in heavily pretreated HL patients. However, galiximab was well tolerated [98].
7.8 Therapeutic Efficacy of Cytokines
Targeting cytokines in immunotherapy for HL is restricted due to a variety of challenges. Since structural properties, binding avidity, and retention time in the tumor tissue as well as the pharmacodynamics and pharmacokinetics are all affected by the optimal design of each individual domain in the fusion protein, an optimized molecular design is mandatory for efficient treatment with antibody-targeted cytokines [7]. HL sheds the targeted cell surface antigen CD30 in substantial amounts which competes with the tumor cell-bound Ag in binding, leading to substantial rise in serum levels of soluble CD30 (sCD30). Therefore, greater affinity to the solid-phase-bound Ag in the presence of high amounts of soluble Ag is needed. In addition, high systemic toxicity is observed with the administration of some cytokines, hence limiting their application for specifically targeted tumor tissues. As a result, when they are delivered by a targeting antibody fused to the cytokine, healthy tissues are left free from toxic cytokine concentrations. On the other hand, fusion to other protein domains may decrease functional properties of cytokines. Hence, the potentiality of the Ab-targeted cytokine depends on the binding avidity and the immunomodulatory capacity of the fusion protein [7]. Various cytokines are applied in this regard which are discussed herein.
7.8.1 Interleukin-2 (IL-2)
The first clinical trial on the administration of recombinant IL-2 (rIL-2) was conducted in 1984. Thereafter, several clinical trials have been conducted to examine the efficacy of rIL-2 with or without LAK cells in patients with refractory HL. Even though previous clinical trials have approached patients with relapsed or refractory HL which had poor prognosis, the results seemed promising. More recent studies have approached those requiring maintenance regimen alone or combined with other cytokines after high-dose chemotherapy. Intensification of immune-mediated effector mechanism holds great potential in reducing relapse rates after peripheral blood stem cell transplantation. Side effects were those expected from IFN-α and IL-2 when given as single agents, including fever, chills, fatigue, flu-like symptoms, anorexia, nausea, vomiting, and diarrhea which were transient and reversible. Prospective randomized studies are required to confirm the promising results of combined IFN-α/r-IL-2 maintenance therapy after autologous bone marrow transplantation. The application of low-dose IL-2 expands and activates NK cells in both animal models and cancer patients [99, 100].
A therapeutic whole-cell vaccine consisting of IL-2 adsorbed onto aluminum hydroxide as cytokine-depot formulation exhibited potent antitumor immunity, induced delayed tumor growth, controlled tumor dissemination, and led to longer survival in mice challenged with A20 lymphoma. It proved to overcome the adverse effects of intratumoral Treg cells. However, clinical studies are mandated [101].
7.8.2 An IL-2-IL-12 Fusion Protein Targeting Hodgkin Lymphoma
It is hypothesized that an IL-12 polymorphism concomitant with Th2 polarization leading to decreased IL-12 production is a determinant of increased susceptibility in young adult HL [102]. Low IL-12 levels lead to reduced cellular immune response during the disease [103]; therefore, targeting IL-12 may reverse the situation locally in lymphoma lesions. In addition to the crucial role of IL-12 in stimulating cytotoxic T-cell and NK cell activities, it plays a predominant role in Ag processing and presentation. As a result, immunotherapies targeting IL-12 are considered to induce a strong cell-based immune response with enhanced tumor cell killing [104, 105] and an efficient antitumor response [106, 107]. Since a major limitation of current immunotherapy regimens is unintended activation of effector cells beyond target sites, attempts have been made to overcome this inadequacy. T and NK cells express upregulated amount of IL-2 and IL-12 receptors and transient CD30. Therefore, binding of anti-CD30 fusion proteins and unintended off-target effector cell activation may occur. Remarkably, the Ab-binding domains of these proteins bind to tumor cells with higher avidity, and these specific bindings are more resistant to blocking by soluble target Ag. Lines of evidence suggest that by simultaneous targeting of cooperating cytokines, a broad immune response is activated, hence providing a valuable response in cancer immunotherapy [7]. An overlapping activity in mustering T and NK cells has been described between IL-2 and IL-12 [108]; IL-2 leads to potent proliferation induction, while IL-12 stimulates cytokine secretion including IFN-c. A synergic effect between these two cytokines with respect to these functions is manifested, resulting in more efficient lysis of target cells [108]. In order to mobilize both adoptive and innate immune cells for an antitumor attack, IL-2 and IL-12 have been fused to an anti-CD30 scFv Ab; hence both cytokines are accumulated on the malignant CD30+ HRS cells in the lymphoma lesion [7]. This dual cytokine-antibody fusion protein has revealed significant activity and superior efficacy in activating resting T and NK cells compared to the corresponding fusion proteins containing either IL-2 or IL-12 in mouse model [108]. Cytolysis in resting NK cells and reactivated IL-2-deprived T cells was induced by this dual cytokine protein, benefiting immunotherapy of HL. Interestingly, simultaneous application of both single-cytokine proteins was less effective in delivering both cytokines to the same cell at the same time. Using this technique facilitated dimerization of the molecule via integration of various domains, thus leading to favorable binding to solid-phase Ag, even in the presence of the soluble antigen, as well as higher and specific retention in the targeted tissues and lower tissue penetration in vivo studies. Overall, dimerized fusion proteins appear to be more suitable for site-specific immunotherapy compared to the corresponding monomeric proteins [7]. Due to the predominant expression of CD30 on activated Th2 cells [109] which are present in high numbers in the tissue of HL [110], the tumor environment of HL may be modulated by shifting T cells to Th1 reactivity by the application of anti-CD30-IL-12-IL-2 dual cytokine fusion protein. In addition, both types of cytolytic effector cells, T and NK cells, may be reactivated by simultaneous synergistic action of IL-2 and IL-12. It is hypothesized that IL-2 and IL-12 lead to NK and T-cell activation followed by increased IFN-c secretion and shift the Th2 imbalance in the lymphoma lesion toward Th1 reactivity; therefore counteracting T-cell hyporesponsiveness in HL [111].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree