General Aspects of Immunosenescence
The immune system undergoes profound age-related changes, collectively termed immunosenescence. This process affects various cell types including hematopoietic stem cells (HSCs), lymphoid progenitor cells in the bone marrow and in the thymus, the thymus itself, mature lymphocytes in peripheral blood and secondary lymphatic organs and also elements of the innate immune system. These immunological changes contribute to elevated susceptibility to infectious diseases, to more severe symptoms, prolonged duration and poorer prognosis of infections and to decreased protective effects of vaccinations. Reactivation of Varicella-Zoster virus leading to herpes zoster is observed by far more frequently in elderly compared to young adults. Infections with influenza virus are associated with more severe symptoms in elderly patients and with an increased risk for secondary complications. Additionally, risks for many other infections are increased in elderly people. As the elderly population is particularly susceptible to infection and vulnerable in case of disease, vaccination is of special importance. Unfortunately, the efficacy of various vaccines, e.g., influenza, hepatitis A and hepatitis B, is lower in elderly people. New strategies are needed to improve vaccination and to develop vaccines that specifically target and stimulate the aged immune system.
Effect of Aging on Different Cell Types
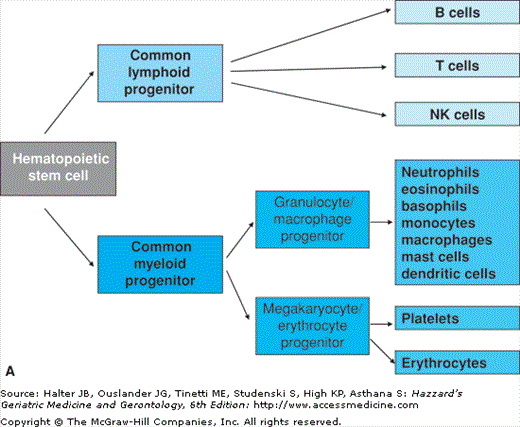
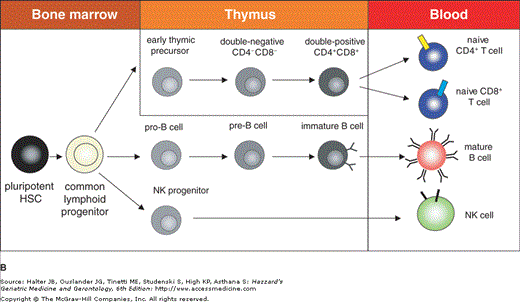
All circulating blood cells of an adult individual including immature lymphocytes are generated in the bone marrow. Both liver and spleen can also be recruited as sites for haematopoiesis in case of increased need for newly generated blood cells or if the bone marrow is injured. All blood cells originate from a common stem cell (HSC) and become committed to develop along particular lineages. The first step of differentiation leads to the separation of myeloid and lymphoid progenitors. Myeloid progenitors further differentiate to become erythrocytes, platelets, basophils, eosinophils, neutrophils, monocytes, macrophages, mast cells, or dendritic cells (DC). Lymphoid progenitors give rise to B lymphocytes, T lymphocytes, and natural killer (NK) cells (Figure 3-1A). Maturation of B cells, which includes rearrangement and expression of immunoglobulin genes as well as selection for cells with functional immunoglobulins and against self-reactive B cells, takes place in the bone marrow. Mature B cells then enter the circulation and lymphoid organs where they encounter their specific antigens. T lymphocytes leave the bone marrow in an immature state and migrate to the thymus where maturation with somatic recombination and expression of the T cell receptor (TCR) genes occur. Additionally, positive selection for functional TCRs and negative selection against self-reactive T cells take place in the thymus. Like B cells, mature T cells finally migrate to lymphoid organs and are also found in the blood stream (Figure 3-1B).
Figure 3-1.
Hematopoiesis and maturation of lymphoid cells. (A) Schematic representation of hematopoiesis. Hematopoietic stem cells differentiate into lymphoid and myeloid progenitors that give rise to the different cell types found in peripheral blood. (B) Maturation of lymphoid cells. Maturation of T cells takes place in the thymus, whereas B cells and NK cells undergo maturation in the bone marrow. All mature lymphocytes migrate into the periphery and can be found in blood.
The overall amount of hematopoietic tissue in the bone marrow is decreased with age. Changes of the hematopoietic microenvironment and alterations of hormone production disturb self-renewal and lineage commitment of HSCs. HSCs themselves are also affected by aging as indicated, e.g., by shortening of telomeres. In combination, these two effects may lead to fewer and less functional HSCs with increasing age. Interestingly, the lymphoid lineage is far more compromised by age-related changes than the myeloid lineage. Fewer pro-B cells are generated in aging and fewer of these cells transit through the next differentiation steps. As a consequence, the number of mature B cells leaving the bone marrow is lower. T cell precursors seem to be less affected by aging. However, due in part to dramatic alterations in the thymus, where the maturation of T cells takes place, the T cell compartment undergoes substantial changes with age.
Maturation of T cells takes place in the thymus, a bilobed organ situated in the upper anterior thorax, just above the heart. It is divided into multiple lobules that consist of an outer cortex that is densely populated with thymocytes and an inner medulla that is sparsely populated with thymocytes. Other cell types found in the thymus are nonlymphoid epithelial cells, marcrophages, and lymphoid DC. They provide important stimuli (e.g., cytokines and physical interaction via major histocompatibility complex (MHC) molecules) for the development of mature T lymphocytes. Immature T cells enter the thymus where they proliferate and differentiate into mature T lymphocytes. During this differentiation, the T cells migrate from the outer cortex to the medulla from where they exit the thymus and spread to the periphery. The very complex maturation process includes proliferation, recombination of the TCR genes to assure diversity of the T cell repertoire, negative selection, and extensive apoptosis of self-reactive or nonreactive T cells and positive selection of expedient T cells, which recognize foreign antigens presented by MHC complexes. The T cells that exit from the thymus are called naïve as they have never had contact with their specific antigen. Naïve T cells make up the reservoir of cells that is needed to respond to neoantigens that are encountered throughout life.
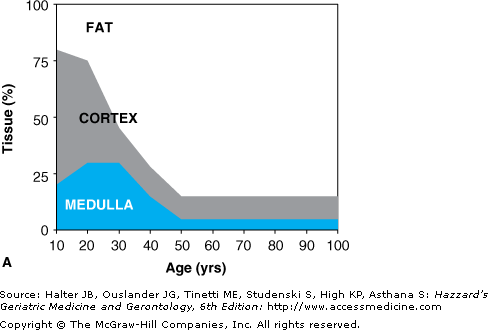
One of the hallmarks of immunosenescence is the involution of the thymus, which is characterized by a reduction in the overall size of the organ and by a replacement of the functional cortex and medulla tissue by fat. These changes start early in life and are almost complete by the age of 40 to 50 years. Figure 3-2 illustrates the redistribution of tissues in the thymus with increasing age and depicts histological changes. Involution of the thymus could be caused by both defects in the T cell precursor cells and changes of the thymic stromal cells. This working model postulates that these changes could occur simultaneously in each compartment or one could precede the other. In either case, age-associated degeneration of the one compartment negatively influences the other, leading to progressive loss of thymic function. As a consequence, the number of thymocytes and of naïve T cells exiting the thymus is dramatically decreased with age. The missing output of newly generated naïve T cells leads to crucial changes in the T cell repertoire and the composition of the T cell compartment. However, the overall role of thymus involution in age-related changes of peripheral immune cells remains to be determined.
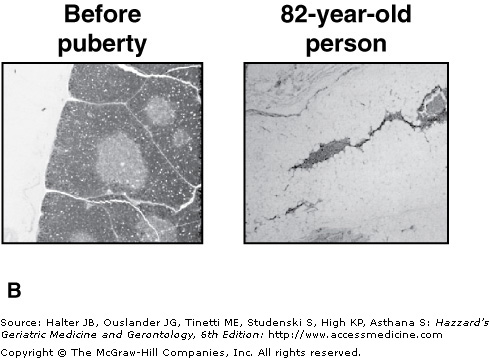
Figure 3-2.
Thymic involution and loss of functional thymic tissue. (A) With increasing age, functional thymic tissues (medulla and cortex) are replaced by fat. Depicted are percentages of thymic tissues and fat tissue against donor age. (B) Histological pictures of human thymic tissue derived from a child or an 82-yr-old person stained with hematoxylin. In the pediatric thymus areas of cortex (outer darker staining) and medulla (inner lighter staining) tissue are prominent. Thymic tissue from elderly individuals shows dramatic reduction of functional tissue and invasion of adipose tissue. Magnification = 120×. Reprinted from George AJT, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol. Today. 1996;17;267. With permission from Elsevier.
The innate immune system is the first line of defense against infection. It consists of cellular and biochemical defense mechanisms that respond to infectious agents by recognizing common structures shared by pathogens. It is not able to distinguish between fine differences of antigens. The innate immune system reacts faster than the adaptive immune system. However, due to a lack of memory mechanisms, the innate immune system is not able to “remember” antigens. There are therefore no differences between primary and subsequent responses to the same pathogen. The major components of innate immunity are physical barriers (epithelia), phagocytic cells (neutrophils, macrophages, DCs), NK cells, the complement system, and a variety of cytokines that regulate and coordinate innate immune responses (Table 3-1). Toll-like receptors (TLR) are highly conserved receptors that are expressed on different cell types of the innate immune system and recognize distinct classes of pathogens. To date, over 20 mammalian TLR have been identified recognizing, for example, lipopolysaccharide (LPS), other bacterial proteoglycans, or unmethylated CpG nucleotides found in bacteria or viruses. Each TLR seems to be crucial in the responses to distinct classes of infectious pathogens. Binding of microbial components to the TLR leads to activation of innate immune cells and induces production of cytokines.
COMPONENT | FUNCTION |
|---|---|
Epithelial barriers | Prevention of microbial entry |
Complement | Opsonization of microbes, killing of microbes, activation of leukocytes |
Mannose-binding lectin | Opsonization of microbes, activation of complement |
Cytokines | Inflammation, activation of phagocytes, stimulation of IFN-γ production |
Dendritic cells | Antigen uptake in peripheral sites, antigen presentation in lymph nodes |
Neutrophils | Phagocytosis, killing of pathogens |
Eosinophils | Killing of antibody-coated parasites |
Mast cells and basophils | Release of granules containing histamine and active agents, induction of inflammation and tissue responses |
Macrophages | Phagocytosis, killing of pathogens, cytokine release, antigen presentation |
Natural killer cells | Release of lytic granules, killing of infected cells and tumor cells |
The main function of neutrophils and macrophages is to recognize, internalize, and destroy pathogens. Macrophages also process foreign proteins to peptides that are then presented to T lymphocytes. This link between innate and adaptive immunity will be described in more detail in the next section. DC are of crucial importance for the initiation of an adaptive immune response to new antigens. They are found under the epithelia of many organs where they capture antigens, process them, and present them to naïve T cells after migrating to lymph nodes.
NK cells are able to directly kill target cells by releasing perforin and granzymes. Perforin creates pores in the target cells through which granzymes enter the cells. These enzymes activate caspases and induce apoptosis of the cell. NK cell function is regulated by a balance of activating and inhibiting receptors. Activation is induced by molecules that are expressed on infected or otherwise stressed host cells. NK cells are thus able to identify, for example, virus-infected cells. MHC class I molecules, which are present on all host cells, act as inhibitory signals and prevent killing of healthy cells by NK cells. As a variety of viruses induce down-regulation of MHC class I in order to avoid recognition by the adaptive immune response, the lack of MHC expression indicates viral infection and is also used by NK cells to identify these cells.
Additionally, cells of the innate immune system produce cytokines that cause inflammation, induce the release of inflammatory plasma proteins, and activate innate and adaptive immune cells, which are recruited to the site of infection (see Chapter 4).
AGE-RELATED DECREASE | AGE-RELATED INCREASE | |
|---|---|---|
Neutrophils | Phagocytic capacity Oxidative burst Bactericidal activity | Membrane viscosity |
Macrophages | Phagocytic capacity Oxidative burst MHC class II expression | |
NK cells | Cytotoxicity Production of proinflammatory cytokines and chemokines Proliferative response to IL-2 | Number of cells |
Cytokines and chemokines | Serum concentration of IL-6, IL-1β, TNF-α Serum concentration of MIG (CXCL9) and IP-10 (CXCL10) |
While most humoral components of the innate immune system such as the complement system are not affected by the aging process, age-related changes of innate immune cells occur, leading to alterations in cell function and in the production of cytokines and chemokines. The overall number of neutrophils remains constant, but there are characteristic age-related functional changes. Both their phagocytic capacity as well as their efficiency of killing internalized microbes is decreased in old age. This leads to an overall reduction of bactericidal activity. A decreased ability to kill pathogens has also been observed in aged macrophages. Additionally, their antigen-presenting function is impaired due to a decreased expression of MHC class II molecules, leading to defects in the activation of CD4+ T cells. Alterations in the expression and function of TLRs also add to age-related impairments within the innate immune system.
NK cell numbers increase with age, but their cytotoxic potential and the production of cytokines decrease on a per cell basis. Proliferation of aged NK cells in response to stimulatory cytokines like IL-2 is reduced. How DC are affected by age is still not fully understood. In aged mice and humans, the number of DC in the skin is decreased and the capacity of DC to migrate to lymph nodes after antigen contact is impaired. Little is known about age-related functional changes of human DC. Animal models suggest that antigen presentation is decreased in old age; however, so far this has not been confirmed in humans. DC differentiated in vitro from monocytes derived from aged humans are indistinguishable from their young counterparts.
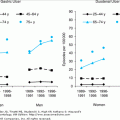
Subclinical inflammatory processes gradually increase with age in a variety of species (see Chapter 4). Elevated plasma concentrations of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-alpha (TNF-α) have been described in elderly populations and were postulated as predictive markers of functional disability and mortality. This progressive proinflammatory state in elderly persons has been referred to as “inflamm-aging.” Such chronic inflammatory processes may support the development and progression of age-related diseases, such as osteoporosis, neurodegeneration, and atherosclerosis. At first sight, “inflamm-aging” seems to contradict the functional defects observed in innate immune cells. However, it is believed that chronic subclinical inflammation is caused by chronic stimulation of the innate immune system by products of degradation processes and/or by the partial inability of the aged immune system to eliminate certain pathogens. This could lead to chronic, yet inefficient innate immune responses.
Genetic factors, like polymorphisms in genes encoding for cytokines like IFN-γ, IL-6, and IL-10 can also influence the severity of inflammatory responses in old age. Chemokines, which direct the movement of circulating lymphocytes to sites of injury of infection, play a crucial role in initiating adaptive immune responses and in directing movement of immune cells through the body. Approximately 50 human chemokines have been described. Alterations in serum concentrations of some of them have been reported in elderly people. However, for most of the proteins studied, conflicting results have been obtained. Some reports show increased concentrations of the proinflammatory, IFN-γ inducible chemokines MIG (CXCL9) and IP-10 (CXCL10). Elevated levels of MCP-1 (CCL2), which also acts proinflammatory by regulating T cell and monocyte recruitment toward sites of inflammation, have been described in elderly people; however, other studies were not able to confirm these findings.
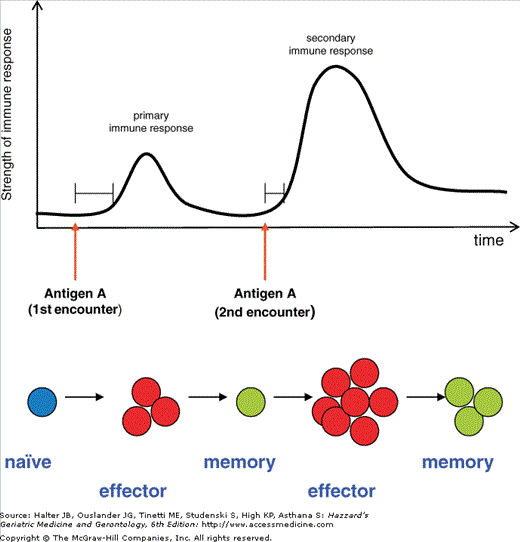
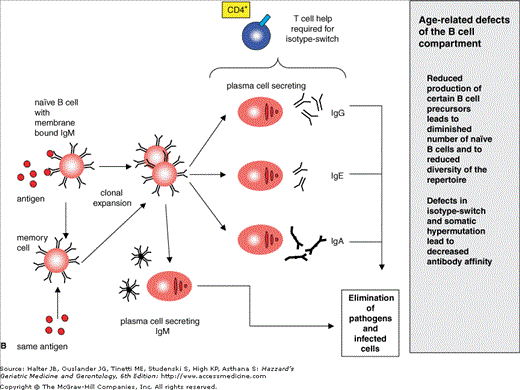
The adaptive immune system consists of lymphocytes, namely, T and B cells, and antibodies, which are produced by B cells. Both B and T lymphocytes can be divided into naïve, memory, and effector cells with regard to their differentiation status. Lymphocytes that have not yet encountered their specific antigen are called naïve. Upon contact with their antigen, these cells proliferate and differentiate into memory and effector lymphocytes. This antigen-triggered response increases in magnitude and effectiveness with each encounter of a particular infectious agent (Figure 3-3). After the first encounter with the antigen, it takes several days for the adaptive immune response to fully respond and activate effector cells. Once the pathogen is cleared, most of the activated effector cells are eliminated. However, long-lived memory cells remain in the body and ensure that the response to a second contact with the same antigen is faster and more pronounced compared to the primary response. Both T and B lymphocytes recognize their antigens via highly diverse surface molecules called T cell receptor (TCR) or B cell receptor (BCR), respectively. The diversity of these molecules is due to extensive somatic recombination of the genes encoding the TCR or BCR during the maturation of lymphocytes. The total number of specificities of the lymphocytes in an individual, called the immunological repertoire, is estimated to be 107 to 109.
Figure 3-3.
Primary and secondary immune response. The upper panel shows a schematic depiction of the strength of the immune response as measured, e.g. by antibody titers or numbers of effector T cells. The secondary immune response after repeated encounter with the same antigen is faster and more pronounced compared to the primary immune response. After repeated stimulation with the same antigen immune responses are sustained at a higher level. The lower panel depicts the expansion and differentiation of lymphocytes in the course of primary and secondary immune responses. Naïve cells differentiate into effector cells after encountering their antigen. Most effector cells are eliminated by apoptosis after clearance of the pathogens. Few cells remain in the body as long-lived memory cells and are able to quickly and efficiently differentiate into effector cells in case of a second contact with the same antigen. After clearance of the pathogens, most effector cells are again eliminated; however, more long-lived memory cells are generated.
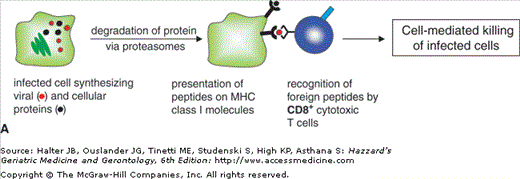
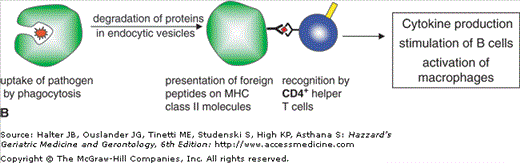
T cells can be divided into CD4+ and CD8+ T cells. CD4+ T cells are called T-helper cells and are required for stimulation of B cells and activation of macrophages. CD8+ T cells or cytotoxic T lymphocytes (CTL) directly interact with virally infected and tumor cells and kill these target cells. T cells are not able to directly recognize foreign antigens. Their highly diverse T cell receptors interact with molecules of the MHC that are associated with peptides derived from antigenic proteins. MHC class I molecules are present in all nucleated cells. All proteins that are synthesized within a cell are processed into peptides and presented via MHC class I on the surface of the cells. CD8+ T cells interact with the MHC–peptide complexes and distinguish between self and nonself peptides. Cells presenting foreign peptides, e.g. viral components on infected cells, are targeted for killing (Figure 3-4A). CD4+ T-helper cells recognize peptides in the context of MHC class II molecules. These proteins are present only on specialized antigen-presenting cells of the innate immune system (monocytes, macrophages, DC) and on B cells. Antigen-presenting cells recognize foreign antigens, internalize them, and process them into peptides, which are presented in the context of MHC class II molecules. Interaction of antigen-presenting cells with specific T-helper cells induces cytokine production in T cells and stimulates B cells to produce antibodies (Figure 3-4B).
Figure 3-4.
Processing and presentation of antigens via MHC class I or class II. (A) Synthesized proteins are degraded via proteasomes and presented on MHC class I molecules. CD8+ cytotoxic T cells recognize foreign peptides and mediate killing of infected cells. (B) Pathogens are internalized by antigen presenting cells; proteins are degraded in endocytic vesicles and presented on MHC class II molecules. CD4+ helper T cells recognize foreign peptides and stimulate B cells and macrophages.
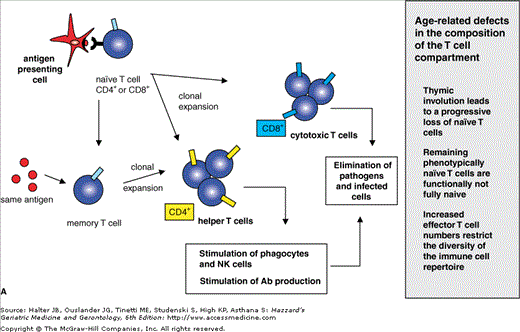
When naïve T cells (both CD4+ and CD8+) make contact with cells presenting their specific antigen via MHC molecules, they proliferate and differentiate into memory and effector T cells. This process is called clonal expansion. As mentioned above, CD8+ and CD4+ T cells exert distinct effector functions. As described above for NK cells, effector CD8+ T cells also release perforin and granzymes to induce apoptosis in infected or tumor cells. CD4+ effector cells stimulate phagocytes of the innate immune system. NK cells induce cytokine production in T cells to enhance immune responses and are required for efficient antibody production by B cells (Figure 3-5A). On the basis of their cytokine profile and effector functions, TH1 and TH2 subpopulations have been characterized within the T-helper cells. Both subsets differentiate from the same precursors, namely naïve CD4+ T cells. The TH1 differentiation pathway is mainly triggered by the cytokine IL-12, which is produced by innate immune cells in response to microbes that infect or activate macrophages and DC. TH1 cells produce proinflammatory cytokines, such as IFN-γ and TNF-α. These cytokines stimulate the microbicidal activity of phagocytes and the production of opsonizing and complement-fixing IgG antibodies. Microbes can then be efficiently phagocytozed and eliminated by phagocytes.