The lung is an important and vulnerable target in immunologic diseases. Not only does the lung participate in systemic immunopathologic processes, but it is also capable of initiating local immune responses that may be beneficial or adverse to the host. With the exception of asthma, primary and secondary immunologic lung diseases are discussed in this chapter according to their presentation, immunologic features, pathologic features, diagnostic criteria, differential diagnosis, treatment, and prognosis. These various entities are either known or proposed to have immunologic mechanisms and include hypersensitivity pneumonitis, allergic bronchopulmonary aspergillosis (ABPA), eosinophilic lung diseases, antiglomerular basement membrane syndrome, Wegener’s granulomatosis, sarcoidosis, idiopathic pulmonary fibrosis (IPF), nonspecific interstitial pneumonia, and cryptogenic organizing pneumonia (COP) of the lung.
HYPERSENSITIVITY PNEUMONITIS
Hypersensitivity pneumonitis (extrinsic allergic alveolitis) is a common and well-studied immunologically mediated lung disease. It occurs after the inhalation of organic dusts and is an IgG-mediated response. The result is a diffuse pulmonary process consisting of reticulonodular or alveolar processes (or both) with poorly formed granulomas. In contrast to other granulomatous diseases (e.g., sarcoidosis, coccidioidomycosis), the immunopathologic process is localized in the lung, and there is no systemic involvement. Moreover, it can fully remit if the antigenic stimulus is removed. Hypersensitivity pneumonitis develops in only 5% to 15% of the exposed population, and the majority of patients are nonatopic and nonsmokers. The antigenic materials may be of animal, vegetable, fungal, bacterial, or chemical origin. Reactions can be classified as acute or chronic. In general, there is no age, sex, or significant geographic predilections other than those related to specific occupational exposures.
I. CLINICAL PRESENTATION
The antigens listed in
Table 10-1 are recognized as capable of sensitizing susceptible persons and subsequently causing hypersensitivity pneumonitis. This is only a partial list, and new sources from occupational exposure, homes, and hobbies are reported annually. Despite this long expanding list, these inciting antigens have striking similarities in their clinical, radiographic, and pathologic outcomes.
A. Acute hypersensitivity pneumonitis occurs when exposure is heavy but intermittent.
1. Symptoms and signs. Acute symptoms, including fever, chills, dyspnea, chest tightness and dry cough, appear 4 to 6 hours after each exposure and remit when the agent is avoided. This is considered a late, or Arthus (type III), immune reaction. Physical exam reveals fever, tachypnea, tachycardia, and a few lung rhonchi or crackles.
2. Laboratory findings. Laboratory tests are of limited utility. Peripheral neutrophilia (without eosinophilia) and increased IgG levels, including antigen-specific IgG, are common in the acute form. Serum IgE levels are usually within normal limits. Nonspecific markers of inflammation such as an elevated ESR, C-reactive protein, and rheumatoid factor may also be present.
3. Radiographic findings. Radiographic abnormalities will typically develop with repeated antigen exposure but may not parallel the severity of disease. Up to 4% of patients may have normal x-rays, while up to 45% may have very subtle changes. Initially, diffuse airspace opacification is present on x-rays. This resolves into a fine nodular or reticulonodular pattern. These changes may be completely reversible over 4 to 6 weeks if the initiating exposure is avoided. High-resolution computed tomography (HRCT) has a higher sensitivity and specificity for detecting lung involvement and may be useful in cases where routine chest x-rays show only subtle findings or are normal. Findings on HRCT include scattered, small, rounded opacities in a centrilobular distribution as well as patchy airspace opacification.
4. Physiologic tests. Pulmonary function tests show hypoxemia and a restrictive ventilatory defect, with reduced vital capacity, total lung capacity, diffusing capacity, and static compliance. Airway obstruction is not typical unless the patient is atopic or has a concurrent obstructive pulmonary disease. Nonspecific airway hyperreactivity can be seen.
B. Acute hypersensitivity pneumonitis in asthmatic patients. Approximately 10% of patients with hypersensitivity pneumonitis have atopy and asthma. A two-stage reaction develops in these patients if they are exposed to organic dust. The immediate asthmatic, or type I, immune reaction will be manifested by dyspnea, wheezing, and an obstructive ventilatory defect. This reaction subsides and is followed in 4 to 6 hours by a type III immune reaction.
C. Subacute or intermittent HP
1. Symptoms and signs. Subacute hypersensitivity pneumonitis is characterized by the gradual development of productive cough, dyspnea, fatigue, anorexia, and weight loss. Physical exam typically reveals tachypnea and diffuse rales.
2. Laboratory findings. The major laboratory abnormality is lymphocytosis in bronchiolar lavage fluid.
3. Radiographic findings. As in acute HP, chest radiography can appear normal or show micronodular or reticular opacities. Abnormalities on HRCT include diffuse micronodules, ground-glass attenuation, focal air trapping, and with more prolonged disease cicatricial emphysema or mild fibrotic changes. These findings also undergo dramatic improvement with treatment with glucocorticoids or with removal of the antigen.
4. Physiologic testing. Pulmonary function testing typically has a restrictive abnormality or a mixed obstructive and restrictive pattern. The diffusion capacity (DLCO) is also reduced in most cases.
D. Chronic hypersensitivity pneumonitis occurs when the exposure is mild but more continuous (e.g., from a single parakeet).
1. Symptoms and signs. Progressive dyspnea, decreased exercise tolerance, productive cough, and weight loss develop insidiously. Acute episodes of chills and fevers are less likely. Wheezing, bibasilar crackles, cyanosis, clubbing, and cor pulmonale develop as pulmonary inflammation and fibrosis progress.
2. Radiographic findings. The diffuse nodular and reticulonodular pattern characteristic of the acute and subacute stages is superimposed on fibrosis and honeycombing and loss of lung volume and compensatory over inflation (emphysema) of the less involved lung zones. These changes are typical of diffuse interstitial fibrosis of any origin and indicate irreversible damage to the lungs. HRCT may be useful for distinguishing hypersensitivity pneumonitis from IPF, which usually exhibits more extensive honeycombing and a predominance of changes in the peripheral and lower lung zones.
3. Physiologic testing. Pulmonary function tests show severe restrictive disease, with variable airway obstruction and air trapping.
II. IMMUNOLOGIC FEATURES
A. Immunopathogenesis. HP is characterized both by proliferation of CD8+ cytotoxic lymphocytes and by an exuberant production of antibody, especially IgG, to offending antigens.
1. Acute phase: macrophage-lymphocyte response. After inhalation of an offending antigen, it binds IgG forming an immune complex, which subsequently initiates a complement cascade resulting in activated macrophages.
These macrophages secrete chemokines and cytokines that first attract neutrophils and, after several hours, attract T lymphocytes and monocytes. Increased numbers of CD4
+ TH1 cells appear in the BAL fluid shortly after exposure, but in most cases of HP, C8
+ cells predominate later. This results in a reversal of the usual CD4
+:CD8
+ ratio (≤1:2) (normal 1.2-1.6:1), which is opposite of the alteration observed in sarcoidosis (CD4
+:CD8
+ ratio ≥ 2:1). These CD8
+ cells are composed of both activated suppressor (CD8+) and cytotoxic (CD8+ and CD56
+) lymphocytes.
2. Subacute phase: granuloma formation. After recruitment into the lung and activation, the young macrophages develop into epithelioid cells and multinucleated giant cells. Lymphoid follicles containing plasma cells also develop in the lesions during the subacute phase.
3. Chronic phase: fibrosis. Early collagen formation by myofibroblasts occurs, and the extracellular matrix surrounding the granuloma becomes rich in the proteoglycan versican. Activated alveolar macrophages express increased amounts of TGF-ß, a potent stimulator of fibrosis and angiogenesis
B. Serum precipitins. The characteristic immunologic feature of hypersensitivity pneumonitis is the presence of precipitating (usually IgG) antibody to the offending antigen. Serum antibodies or precipitins are readily and reproducibly demonstrated by the Ouchterlony double immunodiffusion technique. However, the presence of serum precipitins reactive to an antigen present in the patient’s environment is not prima facie evidence that it is the causal antigen in hypersensitivity pneumonitis. Although the positive precipitin test is clinically helpful, it actually indicates prior exposure and sensitization and does not necessarily correlate with clinical sequelae. Precipitins are observed in 90% of patients with active farmer’s lung, but the percentage with detectable antibodies falls as time passes. Serum precipitins without clinical pneumonitis can develop in up to 50% of exposed asymptomatic patients. Conversely, there are rare patients with clinical disease and no demonstrable antibodies. Antibodies can be demonstrated by more sensitive techniques such as ELISA, immunoelectrophoresis, and immunofluorescence, although their specificity is lower. The correct antigen must be used to detect the antibodies, but many causative antigens have not been identified. Thus, a negative precipitin test in the face of convincing clinical evidence does not exclude the diagnosis, whereas a positive test without appropriate clinical findings does not establish a diagnosis.
C. Skin testing. Antigens for thermophilic actinomycetes and many molds result in a nonspecific irritant response that interferes with skin testing. Cutaneous anergy may also develop from increased suppressor T-cell activity. Many antigens provide a high percentage of positive responses even in exposed but unaffected individuals. Skin testing is therefore neither specific nor sensitive in determining the cause or presence of hypersensitivity pneumonitis. Skin testing for immediate, late-onset or delayed reactions does not currently have a role in diagnosis or management of hypersensitivity pneumonitis.
III. PATHOLOGIC FEATURES
All forms of hypersensitivity pneumonitis have similar and nonspecific pathologic features regardless of the inciting agent. The histologic reflection of precipitating antigen-antibody complexes and activation of the complement
cascade in lung tissue are best demonstrated in early hypersensitivity pneumonitis as the fibrosis seen in the chronic phase is nonspecific. The histology of hypersensitivity pneumonitis includes (1) a mononuclear interstitial infiltration with a bronchocentric distribution (100%); (2) poorly formed noncaseating granulomas surrounding bronchioles (70%); and (3) airway inflammation with foamy macrophages (65%), often associated with bronchiolitis obliterans (50%). Over the course of several months, the histology becomes nonspecific as the granulomas disappear and interstitial fibrosis and obliterative bronchiolitis predominate, resulting eventually in honeycomb cysts and end-stage fibrosis.
IV. DIAGNOSTIC APPROACH
HP can be an elusive diagnosis and challenge even the experienced clinician. As HP can mimic multiple diseases, the diagnosis is founded on a high index of suspicion coupled with suggestive radiographic, physiologic, and immunologic findings.
A. A high index of suspicion is based on a detailed environmental history. Although patients often associate recurrent exposures with symptoms in the acute form of hypersensitivity pneumonitis, the exposure in the chronic form is much more difficult to identify.
B. Chest x-rays, HRCT, and pulmonary function tests consistent with hypersensitivity pneumonitis.
C. Serum precipitins and BAL consistent with hypersensitivity pneumonitis. As stated above, negative studies do not rule out disease, while positive studies do not necessarily confirm a diagnosis. BAL may be done as an adjunct to rule out infection but is not a routine test required for the diagnosis of hypersensitivity pneumonitis.
D. Histologic findings consistent with hypersensitivity pneumonitis. This can usually be done with good transbronchial biopsies directed by radiographic findings, although occasionally (usually in the chronic form) an open lung biopsy is preferred. Special stains and cultures to rule out infectious etiologies should be sent.
E. Trial of avoidance and/or controlled reexposure to the suspected antigen or environment. Deliberate repetition of natural exposure to the suspected antigenic environmental source (e.g., barn, factory) and observing the clinical response (by physical examinations, chest x-rays, and/or spirometry before and after exposure) constitute a simple, relatively safe diagnostic procedure.
F. Allergen inhalation challenge test. When the specific diagnosis remains in doubt because the relevance of a particular exposure is questionable, allergen inhalation or bronchial challenge tests can be used to establish a definitive diagnosis. Various dusts or liquids in the home or workplace can be collected and cultured and extracts prepared from these for inhalation challenge studies in order to identify the responsible antigen. Positive reactions to aerosolized extracts of the appropriate antigen will produce symptoms and signs of hypersensitivity pneumonitis as immediate, late, or dual reactions. However, the patient can become severely ill during the procedure and require hospitalization and parenteral corticosteroids. Thus, this procedure should not be performed routinely and only in laboratories experienced in its administration.
V. DIFFERENTIAL DIAGNOSIS
Both the acute and chronic forms of hypersensitivity pneumonitis can be confused with acute or recurrent pneumonias (viral, fungal, atypical), mycobacterial disease, drug-induced lung disease, organic dust toxic syndrome, ABPA, silo-filler’s disease, pulmonary alveolar proteinosis, sarcoidosis, and collagen vascular disease. The latter two are most often accompanied by systemic, mediastinal, or pleural involvement not found in hypersensitivity pneumonitis. The many causes of interstitial fibrosis, summarized in
Table 10-2, can also be confused with chronic hypersensitivity pneumonitis. Although the history and physical as well as radiologic findings can usually differentiate and limit the possibilities, open lung biopsy is justified in puzzling cases or patients without a correlating environmental history.
VII. PROGNOSIS
Provided the antigen is avoided and/or corticosteroid therapy is initiated before irreparable tissue damage (fibrosis) occurs, the prognosis is excellent. In patients with acute disease, avoiding the offending antigen results in a return-to-normal
pulmonary function. However, in chronic disease, pulmonary fibrosis and advanced respiratory failure often exist when the patients first present for treatment.
ALLERGIC BRONCHOPULMONARY ASPERGILLOSIS
ABPA, the most commonly recognized cause of the allergic bronchopulmonary fungoses, represents an exaggerated immunologic response to fungal colonization of the lower airways. Inflammation in the airways results in subsequent central bronchiectasis, poorly controlled asthma, and frequent exacerbations of asthma or cystic fibrosis (CF).
Aspergillus fumigatus is the most common causative agent of ABPA, although other
Aspergillus species and other fungal
organisms have been implicated. The prevalence of ABPA is 2% to 15% in patients with CF, 1% to 2% in patients with asthma, and up to 39% in those asthmatics admitted to the ICU. In the majority of patients, the diagnosis is made after the age of 20 years. The inflammation includes immediate hypersensitivity (type I), antigen-antibody complexes (type III), and eosinophil-rich inflammatory cell response (type IVb).
I. CLINICAL PRESENTATION
A. Symptoms and signs. The symptoms from the airway inflammation may be difficult to separate from the underlying difficult-to-control asthma or CF. In fact, with the development of ABPA, the symptoms of asthma or CF typically worsen and may manifest with a new or worsening cough or an increase in sputum production or wheezing. Thick mucus that is tenacious and resistant to suctioning is common. Patients may expectorate brownish plugs or flakes (30% to 60%) and, occasionally, bronchial casts. Patients may variably present with systemic symptoms such as fever, malaise, and weight loss. Physical exam may reveal wheezing or evidence of lobar or segmental collapse from mucous plugging.
B. Laboratory Findings
1. Peripheral eosinophilia. Peripheral blood eosinophilia (>1,000/mm3) is common; however, a low eosinophil count does not exclude ABPA.
2. Aspergillus skin test. A type I reaction, erythema and edema developing in 1 to 20 minutes, is a characteristic finding of ABPA and represents the presence of A. fumigatus specific IgE antibodies. A type III reaction, edema within 6 hours, represents the immune complex hypersensitivity reaction.
3. Total serum IgE levels. The total serum IgE level is the most useful test for the diagnosis and follow-up of ABPA. A normal IgE level excludes ABPA from the differential. Total IgE levels decrease with the use of glucocorticoids, and a 35% to 50% decrease in total levels is interpreted as disease remission. A doubling of IgE levels may indicate relapse of disease.
4. Serum A. fumigatus–specific IgE and IgG antibodies. An elevated level of A. fumigatus-specific IgG or IgE antibodies measured by fluorescent enzyme immunoassay (Aspergillus EIA) is characteristic of ABPA, but nonspecific.
5. Serum precipitins against A. fumigatus. The precipitating IgG antibodies are elicited from crude extracts of A. fumigatus and are demonstrated by a double gel diffusion technique. These can be seen in other lung disease and are therefore supportive but not diagnostic of ABPA.
6. Sputum culture. A sputum culture growing A. fumigatus is supportive but not diagnostic for ABPA as the fungus is ubiquitous in the environment and can be seen in other lung conditions.
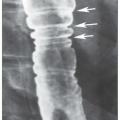
C. Radiographic findings. A wide spectrum of radiographic findings is seen in ABPA. Mucoid impaction of involved airways frequently results in segmental or lobar atelectasis that is visualized as transient or fixed pulmonary infiltrates. Central or proximal bronchiectasis with normal tapering of the distal airways is specific for ABPA. Impaction of dilated bronchi is classically described as “finger-in-glove” projections. Tram tracking, which represents thickened bronchial walls, is also described. HRCT has a higher specificity and sensitivity in detecting central bronchiectasis than plain
x-rays. Common HRCT findings include central bronchiectasis, mucus plugging consolidations, centrilobular nodules with tree-in-bud opacities, bronchial wall thickening, atelectasis, and mosaic perfusion with air trapping on expiration. Upper lobe fibrosis occurs in patients with chronic disease.
D. Physiologic tests. The presence of mucus plugging (mucus impaction syndrome) and airway damage is commonly, but not always, associated with evidence of reversible airways obstruction and lowered diffusing capacity on pulmonary function testing. This may wax and wane depending on the changing mucous obstruction. A restrictive ventilatory pattern may occur with pulmonary fibrosis, as a result of longstanding disease, and may not reverse with corticosteroid therapy.
II. IMMUNOLOGIC FEATURES
Persistence of Aspergillus organisms in the lung results in the formation of hyphae, which release antigens that compromise mucociliary clearance, breach the airway epithelial barrier, and activate the innate immunity of the lung. The result is an influx of inflammatory cells leading to an early- and late-phase inflammatory reaction. These antigens are presented to T cells with resultant activation of Th2 CD4+ T-cell responses. The T-cell response causes a release of cytokines (interleukin [IL]-4, IL-5, and IL-13), which leads to a total and A. fumigatus-specific IgE synthesis, mast cell degranulation, and activation of a strong eosinophilic response. Skin tests positive for Aspergillus show both an immediate wheel-and-flare reaction (type I) and a late reaction of erythema and edema (type III).
III. PATHOLOGIC FEATURES
The pathology of ABPA can vary from patient to patient and even within a single patient. Common histologic findings within affected bronchi include mucus, fibrin, Curschmann’s spirals, Charcot-Leyden crystals, and inflammatory cells (notably eosinophils within noncaseating granulomas, bronchial walls, and peribronchial parenchyma). Although thick, tenacious mucous plugs with fungal elements fill the affected bronchi, the organism does not invade the bronchial wall or lung parenchyma. In some patients, the bronchial wall damage is associated with intense parenchymal infiltration by eosinophils and mononuclear cells and the presence of granulomas (bronchocentric granulomatosis). Subsequently, proximal cystic bronchiectasis and upper lobe fibrosis develop. Bronchiectatic cavities can often show the scant hyphae of Aspergillus. Patients can often show a pattern of organizing pneumonia similar to that of bronchiolitis obliterans.
IV. DIAGNOSTIC APPROACH
There is no individual test to establish the diagnosis of ABPA, and the diagnosis is usually confirmed by the use of clinical, radiographic, and immunologic criteria. Diagnosis may be complicated by recent steroid use, as it will alter skin reactivity to Aspergillus antigens, serum total IgE levels, and peripheral blood eosinophilia levels. If the diagnostic criteria are not met but there is a high level of suspicion for the diagnosis of ABPA, the studies can be repeated when the patient has been off of steroids for a sufficient amount of time.
A. Specific etiologic approach. The diagnosis is certain if the following conditions (usually present in over 90% of the patients) are present. The presence or absence of central bronchiectasis classifies ABPA as Seropositive-ABPA (ABPA-S) or ABPA with central bronchiectasis (ABPA-CB).
1. Asthma (episodic bronchial obstruction) or CF with acute or subacute deterioration
2. Immediate skin test reactivity to Aspergillus antigens
3. Serum total IgE levels > 1,000 ng/mL
4. Elevated specific serum IgE and IgG to A. fumigatus
5. In addition for ABPA-CB: central bronchiectasis
6. Additional findings:
Mucous plugs.
Aspergillus on culture of sputum
Precipitating antibodies to A. fumigatus (may be considered a diagnostic criteria in CF).
Transient or fixed pulmonary infiltrates (for CF a change in radiographic findings may be considered a diagnostic criteria)
Delayed skin test reactivity to Aspergillus antigens.
V. DIFFERENTIAL DIAGNOSIS
Patients with ABPA frequently have had a prior diagnosis of problematic asthma, CF, chronic bronchitis, recurrent pneumonia, tuberculosis, or bronchiectasis from other causes. However, it is possible for some of these entities to coexist with the fungal hypersensitivity. ABPA may be confused with other conditions exhibiting pulmonary infiltrates with eosinophilia (
Table 10-3).