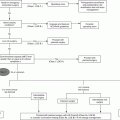
Innate
Macrophage
Reduced phagocytic activity
Reduced generation of nitric oxide
Reduced generation of superoxide
Neutrophil
Reduced phagocytic activity
Reduced generation of superoxide
Dendritic cells
Reduced pinocytosis and endocytosis activity
Reduced phagocytosis of apoptotic cells
Impaired cellular migration
Natural killer cells
Increased total cell number
Reduced cytotoxic ability
Reduced cell proliferation after interleukin-2 exposure
Cytokines
Increased production of interleukin-6
Increased production of TNF-α
Increased production of IL-1β
Adaptive
T cells
Reduced naïve cell population
Increased memory cell population
B cells
Reduced antibody isotype switching
Reduced dendritic cell stimulation
Reduced naïve cell population
Increased memory cell population
Aging of the Innate Immune System
Neutrophil
Neutrophils play a critical role in the acute inflammatory host response. These short-lived phagocytic cells are recruited from the peripheral blood via a gradient of chemokines and cytokines produced locally at the site of infection. Aging per se does not have a known affect on the number of neutrophils in the blood or the number of neutrophil precursors in the bone marrow [1]. Despite their preserved quantity most other aspects of neutrophil function are diminished such as phagocytosis and the generation of reactive oxygen species [2, 3]. There is conflicting data on the effect of aging on chemotaxis with some studies showing no effect [4, 5] and others demonstrating decreased chemotaxis [6–8].
Macrophages
Macrophages have many integral functions in the innate immune system. They function as sentinels for microbes in tissue; through the release of effector molecules, they orchestrate the adaptive immune response and play an essential role in wound healing. Macrophages function as first responders to invading microbes. They reside in numerous tissues, such as Kupffer cells in the liver, microglia in the brain, osteoclasts in the bone, and undifferentiated monocytes in the blood. They detect pathogens by recognizing specific pathogen-associated molecular patterns (PAMPs) present on the microbes. They phagocytize invading bacteria, fungi, parasites, protozoa, and apoptotic cells and destroy them via both oxygen-dependent and oxygen-independent pathways [9].
Although the number of circulating blood monocytes in elderly and young subjects is similar, there is a significant decrease in macrophage precursors and macrophages in the bone marrow [10]. Macrophages in the elderly have reduced levels of MHC class II, which may contribute to poorer T-cell responses [11]. The macrophage’s phagocytic function and its chemotactic ability are also diminished with age [12, 13]. Additionally, the ability of aged macrophages to destroy microbes via products of the respiratory burst is diminished; this impaired bactericidal capacity may increase the duration of infection in the elderly [14].
Natural Killer Cells
Natural killer cells (NK) are responsible for destroying host cells that have been compromised by tumor or viral infection. The number of NK cells increases with age; however, cytolytic activity and production of interferon-γ are decreased [15]. NK cells kill cells directly by releasing perforin and granzymes which activate caspases that induce apoptosis. The loss of cytotoxic ability is thought to occur as the result of decreased perforin secretion and production [16, 17]. Clinically, these NK cell deficits can result in an increased risk of infection, morbidity, and mortality in elderly patients [3, 18].
Dendritic Cells
Dendritic cells serve as a bridge between the innate and adaptive immune systems. Acting as antigen-presenting cells they capture microbes through phagocytosis, process extracellular and intracellular antigens, and migrate to lymphoid tissue to stimulate T cells. Dendritic cells also have a regulatory function as demonstrated by their production of type I interferons in response to viral infection and the TNF-α inducible nitric oxide synthase (iNOS) production to defend against bacterial infection [19, 20]. In addition to eliciting immune response, dendritic cells also provoke immunological tolerance by inducing deletion or anergy, thereby limiting autoimmunity [21, 22].
Aging dendritic cells generated from peripheral blood monocytes have been shown to be deficient in pinocytosis and endocytosis when presented with an antigen challenge [23]. Additionally, dendritic cells from aged patients have an impaired capacity to phagocytose apoptotic cells compared to those of younger patients. These cells also display impaired migration [24]. Phagocytosis of apoptotic cells produces an anti-inflammatory effect by inhibiting proinflammatory cytokines [25]. Clinically, the impaired uptake and inefficient removal of apoptotic cells by dendritic cells from aged patients may result in the inflammation and autoimmunity commonly seen with aging [12, 24].
Aging of the Adaptive Immune System
T Lymphocytes
T lymphocytes, also known as T cells, play a central role in cell-mediated immunity. These cells recognize and eliminate cells that have undergone viral or malignant transformation. T cells are differentiated from B cells and natural killer cells by their expression of a T-cell receptor (TCR) on their surface membrane; this receptor binds to antigen and CD3. Progenitor cells from the bone marrow migrate to the thymus where they undergo a highly selective elimination process based on the ability of the cell’s TCR to recognize major histocompatibility proteins, degree of affinity for normal self antigens, and the magnitude and duration of TCR signaling [26]. There are primarily two types of naïve T cells that leave the thymus: CD4+ helper cells and CD8+ cytotoxic T cells.
T-cell responses are initiated in secondary lymphoid tissues by exposure to dendritic cells that present antigen. T cells that possess the specific antigen are then induced to proliferate and differentiate into effector cells that reenter the circulation from the lymph system and disseminate to the site of infection. After the infection is eradicated, the vast majority of the effector cells are destroyed with only a few cells remaining as long-lived memory cells [27].
Thymus involution reaches its maximal level at age 50. This involves the replacement of the lymphoid component and epithelial matrix of the thymus with fibrous and adipose tissue [28]. The net result of these changes is that generation of naïve T cells is severely compromised beginning at the age of 40 years [29–31]. Additionally, there is also a loss of diversity as a substantial shift from naïve T cell to memory T cell occurs, especially after age 65 for CD8+ T cells. Naïve CD4+ T-cell numbers are well maintained until age 70 after which their numbers begin to contract [32]. Elderly patients tend to rely on memory T cells for their primary T-cell response, which may result in compromised immune response following vaccination [33, 34].
B Lymphocytes
B lymphocytes, or B cells, mature in the bone marrow and function in the humoral immune response. During B-cell development genetic rearrangement of immunoglobulin light and heavy chains occurs to produce the antigen-binding region of the B-cell receptor (membrane-bound immunoglobulin). B cells that react with self-antigen are removed by a process of apoptosis or inactivation in the bone marrow [35]. At this point the B cell enters the peripheral blood and lymph circulation as mature naïve B cells, where antigen activation occurs. Further differentiation is dependent on activation by antigen and signaling from helper T cells [36]. Mature naïve B cells can become either plasma cells, which produce and secrete large quantities of antibodies or memory B cells, which are long-lived cells capable of responding to reactivation by the same antigen.
While the number of precursor B cells and peripheral B cells does not decline with aging, there is a shift toward more antigen-experienced B cells and fewer naïve B cells [37, 38]. There is also a diminished ability for antibody isotype switching resulting in a shift in antibody isotype from IgG to IgM [39]. Isotype switching maintains the same antigen specificity but changes the effector functions of the antibody. Additionally, B cells in elderly patients are less efficiently stimulated by dendritic cells than younger patients due to their relative deficiency in the expression of co-stimulatory molecules CD40 and CD27 [40, 41]. The net effect of these changes is that elderly patients are forced to rely on a B-cell repertoire which lacks optimal diversity and have a low affinity to antigens and are therefore less protective [42].
Inflamm-Aging: Age-Related Subclinical Chronic Inflammation
The aging immune system is characterized by a low-grade, chronic systemic inflammatory state sometimes referred to as “inflamm-aging” [43]. With aging, there is an increased production of proinflammatory cytokines such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-1β [44–47]. This subclinical inflammation may be caused by chronic stimulation of the innate immune system or by the inability of the immune system to eliminate certain pathogens [12].
Increased IL-6 levels are associated with lower muscle mass and strength in healthy elderly adults [48, 49]. Elevated levels of IL-6 and TNF-α have been shown to be associated with increased disability and mortality in community-dwelling elderly adults [48, 50, 51] and an overall increased inflammatory state.
Risk Factors for Infection
Chronic Obstructive Pulmonary Disorder (COPD)
The prevalence of COPD in the US population varies from 2.9 to 14.3 % depending on how COPD is defined [52]. COPD is characterized by airflow obstruction associated with chronic cough, dyspnea on exertion, wheezing, and expectoration [53]. It is the 4th cause of death for all patients 65 and older [54]. The causative agents associated with COPD are cigarette smoking, biomass exposure, and the resulting inflammatory response orchestrated by neutrophils, macrophages, and CD8+ T cells [55]. Exacerbations are common and become more frequent with increasing disease severity [56]. COPD has been shown to be an independent risk factor for developing pneumonia and pulmonary complications after thoracic surgery [57, 58]. Mucosal lesions of the tracheobronchial tree in the presence of mucous hypersecretion promote bacterial adhesion, colonization, and growth that then impede mucociliary clearance. These changes in the histology of the airway in patients with COPD increase the risk for pneumonia [59, 60].
The majority of cases of community-acquired pneumonia (CAP) in COPD patients are caused by Streptococcus pneumoniae, followed by Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, Streptococcus viridans, Coxiella burnetii, and Mycoplasma pneumoniae [61]. COPD patients hospitalized with CAP have higher 30-day and 90-day mortality rates compared to patients without COPD [62].
Additionally, a study of elderly patients older than 65 years undergoing elective surgery for diverticulitis found that patients with COPD had significantly increased odds of developing pulmonary complications (OR 2.2, 95 % CI 1.94–2.50) that were associated with increased morbidity and mortality [63].
Diabetes Mellitus
Increasing age and diabetes work in concert to further weaken an elderly patient’s response to infections. Diabetes is known to increase the risk of surgical site infection (SSI) and nosocomial infections [64–69]. These infectious complications occur in 20–23 % of all patients presenting with postoperative sepsis [70, 71]. Large population studies have concluded that diabetes mellitus increases the risk of cystitis, pneumonia, cellulitis, and tuberculosis [65, 72, 73]. Recent studies have described an association between perioperative and postoperative hyperglycemia with increased risk for an SSI [74–77]. Controlling hyperglycemia has been shown to reduce perioperative nosocomial and wound infections in diabetic patients [77–79].
The increased risk of infection in diabetics is the result of deficiencies in neutrophil and humoral function [80]. Neutrophil functions such as adhesion, chemotaxis, intracellular bactericidal activity, and phagocytosis are impaired [80–84]. Total IgG levels are lower in both uncontrolled diabetic patients [85] and insulin-treated diabetics but not those on oral medications [86]. Furthermore, diabetic patients are less likely to develop a protective antibody response following hepatitis B vaccination [87–89]. Revaccination with one to three additional doses of hepatitis B vaccine can safely increase the proportion of adults that achieve protective antibody levels [90]. The duration of protection against symptomatic and chronic hepatitis B virus infection has been shown to last for more than 22 years in healthy vaccine responders [91]; however, the duration of immunity among persons with diabetes is unknown. Data on vaccine response to influenza is less clear. Diabetic patients have fewer activated lymphocytes but no reduction in antibody response following influenza vaccination [92, 93].
Chronic Kidney Disease and End-Stage Renal Disease
The prevalence of chronic kidney disease in the United States is rising, up from 10 % in 1988–1994 to 13 % in 1999–2004, and is thought to be the result of the increased prevalence of diabetes, hypertension, and an aging population [94]. Infection is the second most common cause of death in patients with end-stage renal disease [95]. Rates of hospitalization for infection are higher for patients with chronic kidney disease for every major organ system than for patients without kidney disease [96, 97]. Chronic dialysis patients often fail to respond to standard vaccination protocols and may require augmented regimens to achieve a protective effect [98–100]. Despite being a high-risk group for infection, vaccination rates for influenza and pneumococcal pneumonia in end-stage renal patients are far lower than recommended [101, 102].
End-stage renal disease and its precursor chronic kidney disease are associated with marked systemic inflammation and diminished immune response [103, 104]. Cytokine dysregulation results from kidney dysfunction, as the kidney is the main route for elimination of cytokines [105, 106]. Uremia causes deficiencies of both the innate and adaptive immune systems [104, 107, 108]. Uremic patients have increased T-cell turnover and apoptosis which leads to a depletion of naïve and memory CD4+ and CD8+ T cells [109–111]. Reduced B-cell proliferation and antibody production are seen in uremic patients [112–114]. Uremia decreases the function of antigen-presenting dendritic cells [115, 116]. Phagocytic function in macrophages and neutrophils is also diminished [117, 118]. Aging further exacerbates these alterations in immune function.
Challenges in Diagnosing Infection in the Elderly
Elderly patients often do not present with pathognomonic signs and symptoms of infection [119, 120]. Cardinal markers of infection such as fever are often absent in older patients. Physiologic changes in the skin cause older patients to conserve less of the body heat they generate. As a result, many noninfected elderly patients fail to achieve a normal body temperature of 37 ºC [121]. Nearly a third of patients over the age of 65 with infection have temperatures below the threshold of fever (38.3 °C), and by age 80 approximately 50 % of patients fail to reach this threshold [122–124].
Nonspecific symptoms such as change in mental status, decline in functional status, failure to thrive, loss of appetite, and incontinence can all be presenting signs of infection [125, 126]. Additionally, cognitive impairment can render older adults incapable of communicating their symptoms to providers. These nonspecific findings are also commonly seen in noninfectious diseases, making the diagnosis of infection in this population challenging.
Microbiology of Infection in the Elderly
Infectious diseases in the elderly are caused by a more diverse group of pathogens than in younger patients [126, 127]. Changes in microbiology may be related to age, comorbid disease, and environmental setting, e.g., community, long-term care, and hospital [60, 120, 128–131]. Long-term care residents are at great risk for infection [132]. There are more than 15,000 long-term nursing facilities serving approximately 1.5 million residents in the United States; of these residents 90 % are older than 65 years [133]. Antibiotic use is common among long-term care residents with durations that can vary dramatically from less than 10 days to greater than 90 days [134]. Low dose and prolonged use of antibiotics select for bacterial resistance [135, 136]. This practice has led to the emergence of highly resistant pathogens among residents at long-term care facilities, which impacts both empiric antibiotic selection and infection control during periodic hospitalizations [137–139].
Common Infections
Urinary Tract Infection
Urinary tract infections (UTI) account for 25 % of community-acquired bacterial infections and 30 % of infections from long-term nursing facilities [140]. Age-related changes contributing to increased risk of UTI include thinning of the mucopolysaccharide layer of the urinary epithelium and reduction in the Tamm-Horsfall protein (THP) in urine that covers type 1 fimbriae on gram-negative bacteria. THP reduces bacterial attachment and deterioration of bladder and urethral function [140–143].
E. coli is the most commonly isolated organism in urine from patients in both community and long-term care facilities. Gram-negative polymicrobial infections with multidrug-resistant organisms are more frequent in residents of long-term care facilities [143, 144]. Microbiologic differences are seen in diabetic patients where E. coli remains the most common causative agent, but to a lesser extent than in nondiabetic patients, greater proportions of Klebsiella species are reported [131, 145, 146]. Enterococci and Staphylococcus species are the most common gram-positive causative agents.
There is a high prevalence of asymptomatic bacteriuria in residents of long-term nursing facilities ranging from 15 to 30 % in men and 25–50 % in females [147]. It is not recommended to initiate antibiotic therapy for asymptomatic bacteriuria, as there is no improvement in survival rate and a tendency toward increased mortality secondary to adverse side effects and superinfection with resistant organisms is observed [148, 149].
The treatment of symptomatic UTI should be based on antimicrobial susceptibility testing. The selection of antibiotic agent is similar to that in the younger community-dwelling population and should take into consideration the local antimicrobial resistance patterns [150].
In patients with a symptomatic uncomplicated UTI, the current recommendations for antibiotic duration are 3–7 days, whereas for more complicated UTI, 10–14 days is appropriate [151, 152]. Men with recurrent UTI require workup for chronic bacterial prostatitis, which could require 6–12 weeks of therapy [150]. For catheter-associated UTI, the Infectious Diseases Society of America suggests 7 days of treatment in patients who have a prompt response and 10–14 days in those who have a delayed response [153].
Respiratory Infections
Influenza and pneumonia ranks as the seventh leading cause of death for patients 65 years and older [54]. In addition to changes in the immune system, age-related changes in oral clearance, mucociliary clearance, respiratory muscle strength, cough reflexes, and lung structure increase the risk for pneumonia in the elderly [120].
A recent 2005 guidelines from the American Thoracic Society and Infectious Diseases Society of America added a new pneumonia category termed healthcare-associated pneumonias (HCAP). Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurs 48 h or more after admission and was not present at the time of admission [154, 155]. In contrast, ventilator-associated pneumonia (VAP) arises more than 48–72 h after endotracheal intubation [155, 156]. HAP and VAP are distinctly different from HCAP which is defined as a pneumonia diagnosed in a patient subjected to one of the following conditions: hospitalized in an acute care hospital for 2 or more days within the past 90 days; residents of a nursing home or long-term care facility; treated with intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days; or received hemodialysis at either a hospital or outpatient clinic [157].
Several studies examining the etiology of community-acquired pneumonia (CAP) have shown Streptococcus pneumoniae to be the most common pathogen, accounting for 20–60 % of cases followed by Haemophilus influenzae (3–10 % of cases) [158, 159]. Other causative organisms include the atypical pathogens Mycoplasma pneumoniae, Chlamydophila psittaci, and Legionella species [160]. Pathogens associated with HAP, VAP, and HCAP differ from CAP, with multidrug-resistant gram-positive and gram-negative bacteria being more common [161].
Summary
An understanding of the aging immune system is vital for those caring for elderly patients sustaining trauma or undergoing emergency operation. The presentation of infection in the elderly may be subtle, yet early identification, source control, and treatment may improve survival. Appropriate use of antibiotics, based upon prior exposure, environment of residence, and likely organisms, is essential. The complex interplay of organ system disease, physiologic changes, and the effects of trauma requires astute attention to the details of the aging patient.
References
1.
Chatta GS, Andrews RG, Rodger E, Schrag M, Hammond WP, Dale DC. Hematopoietic progenitors and aging: alterations in granulocytic precursors and responsiveness to recombinant human G-CSF, GM-CSF, and IL-3. J Gerontol. 1993;48(5):M207–12. Epub 1993/09/01.PubMed
2.
Fortin CF, McDonald PP, Lesur O, Fulop Jr T. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11(5):873–82. Epub 2008/10/14.PubMed
3.
Mahbub S, Brubaker AL, Kovacs EJ. Aging of the innate immune system: an update. Curr Immunol Rev. 2011;7(1):104–15. Epub 2011/04/05.PubMedCentralPubMed
4.
MacGregor RR, Shalit M. Neutrophil function in healthy elderly subjects. J Gerontol. 1990;45(2):M55–60. Epub 1990/03/01.PubMed
5.
Biasi D, Carletto A, Dell’Agnola C, Caramaschi P, Montesanti F, Zavateri G, et al. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20(6):673–81. Epub 1996/12/01.PubMed
6.
Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44(22):1655–64. Epub 1989/01/01.PubMed
7.
Antonaci S, Jirillo E, Ventura MT, Garofalo AR, Bonomo L. Non-specific immunity in aging: deficiency of monocyte and polymorphonuclear cell-mediated functions. Mech Ageing Dev. 1984;24(3):367–75. Epub 1984/03/01.PubMed
8.
Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3(4):217–26. Epub 2004/07/23.PubMed
9.
Verschoor CP, Puchta A, Bowdish DM. The macrophage. Methods Mol Biol. 2012;844:139–56. Epub 2012/01/21.PubMed
10.
Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117(1–3):57–68. Epub 2000/08/26.PubMed
11.
Zissel G, Schlaak M, Muller-Quernheim J. Age-related decrease in accessory cell function of human alveolar macrophages. J Investig Med. 1999;47(1):51–6. Epub 1999/03/11.PubMed
12.
Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–50. Epub 2009/07/25.PubMed
13.
Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–7. Epub 2004/07/23.PubMed
14.
Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76(2):291–9. Epub 2004/03/25.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree