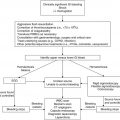
Prior to chemotherapyb
During chemotherapyc
After chemotherapy completionc, d
Diphtheria-tetanus-acellular pertussis
Continuation of primary series during lower-intensity phases of therapy (i.e., ALL in maintenance)
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series
Haemophilus influenzae type b
Continuation of primary series during lower-intensity phases of therapy (i.e., ALL in maintenance)
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series and <5 years of age
Inactivated poliovirus
Continuation of primary series during lower-intensity phases of therapy (i.e., ALL in maintenance)
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series
Pneumococcus
Continuation of primary series during lower-intensity phases of therapy (i.e., ALL in maintenance)
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series and <5 years of age
Hepatitis B
Consider starting immunization series in high-risk settings in seronegativea
Continuation of primary series during lower-intensity phases of therapy (i.e., ALL in maintenance)
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series
Measles-mumps-rubella
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary seriese
Varicella
Consider vaccination during lower-intensity phases of therapy (i.e., ALL in maintenance) in high-risk settingsa
Continuation of primary series; booster 3–6 months after therapy completion in those that finished primary series
Meningococcus
Booster dose for those previously vaccinated; otherwise per routine schedule
Inactivated influenza
Consider if high seasonal incidence
Annually for children ≥6 months of age
Annually for children ≥6 months of age
16.7.1 Diphtheria, Tetanus, and Acellular Pertussis
Two studies were found in regard to response to diphtheria-tetanus-pertussis (DTP) during chemotherapy. Ercan et al. (2005) immunized 17 patients with ALL during maintenance chemotherapy and found no statistical difference compared with 14 healthy controls for tetanus and diphtheria antibody response, although pertussis titers were significantly lower. No adverse reactions were seen. Kung et al. (1984) administered DTP vaccination to 27 children during maintenance chemotherapy for various malignancies and found response to at least 1 of the 2 antigens in 26 of the children (only tetanus and diphtheria were measured for response). Based on their findings, they recommended continuing with the primary vaccination series for inactivated or killed vaccines during maintenance therapy.
16.7.2 Pneumococcal Conjugate Vaccine
In areas without routine pneumococcal vaccination, invasive pneumococcal disease remains a potential risk during and after chemotherapy (Meisel et al. 2007). Allen and Weiner (1981) reviewed 40 episodes of sepsis in 28 children with leukemia and lymphoma and found that 35 % of these episodes were secondary to S. pneumoniae, the most commonly isolated organism. Interestingly, S. pneumoniae was the only organism that caused infection during remission therapy; five of the 40 episodes (12.5 %) were during this time and due to S. pneumoniae. Lehrnbecher et al. (2009) studied 53 children treated for ALL and found persistent lack of protection to pneumococcal antigens which was significantly lower than age-matched, unvaccinated, healthy controls up to 9 months after the completion of therapy (the study period).
Protection during chemotherapy from vaccine strains in those with previous immunization is unknown. Patel et al. (2012) studied 42 children with a history of leukemia ≥6 months off of chemotherapy to assess for serotype-specific antibodies to S. pneumoniae. None of the subjects were noted to have protective antibody concentrations to pneumococcal conjugate vaccine serotypes. Cheng et al. (2012) administered two doses of PCV7 to 44 pediatric oncology patients, including 20 ALL patients in maintenance. Eighty-six to 100 % of patients obtained seropositivity depending on the pneumococcal serotype. No subgroup analysis was reported to determine if differences in seropositivity occurred with different underlying malignancies or based on timing of vaccination. Beyond patients that have received splenectomy as part of their therapy, there is no indication for immunization with the 23-valent pneumococcal polysaccharide vaccine.
16.7.3 Hemophilus Influenzae Type b
In settings with routine vaccination to Hib, invasive disease has plummeted (Bisgard et al. 1998). Risk still remains though in areas without routine immunization (Siber 1980; Feldman et al. 1990). Multiple studies have described the effect of Hib conjugate immunization during chemotherapy (Feldman et al. 1990; Kaplan et al. 1992; Shenep et al. 1994; Cheng et al. 2012). Feldman et al. (1990) vaccinated 50 children with Hib; the overall response rate was 50 %. Shenep et al. (1994) studied 50 children with solid tumors who had not previously been vaccinated for Hib. Seroresponse was noted in 42 % after first vaccination and another 45 % responded to a second dose. Kaplan et al. (1992) studied 18 children with malignancy and found a 50 % seroresponse rate to Hib after 1 immunization. One-third of children responded to a second dose. Weisman et al. (1987) studied 27 children with malignancy, 6 of whom had completed therapy to measure response to Hib vaccination. Eighty-five percent of patients had an appropriate response. Solid tumor patients had a 100 % response; there was no difference in response for those off therapy. Of note, all of these studies were done at a time of significantly decreased chemotherapeutic intensity making it unclear as to their generalizability with modern therapeutic protocols.
16.7.4 Inactivated Poliovirus
The risk of polio is negligible due to the near worldwide eradication of the virus. Only one relevant study by Ogra et al. (1971) could be found, comparing antibody response in patients with leukemia, solid tumors and healthy controls. Response in healthy controls and solid tumor patients was similar while those with leukemia had a blunted response. Again, due to the age of this study, the generalizability to modern therapeutic protocols is unknown. Of note, oral poliovirus (OPV) is contraindicated.
16.7.5 Influenza
16.7.5.1 Inactivated Influenza Vaccine
Although in a Cochrane review Goossen et al. (2009) conclude that there is a paucity of well designed randomized controlled trials to define whether influenza vaccination in children with malignancy during therapy is beneficial considering their blunted response to vaccination, no significant adverse effects were seen in the studies reviewed, and the general consensus is that the benefit of vaccination outweighs cost and any other potential risks, even if seroresponse is blunted (Centers for Disease Control and Prevention 1993; Sung et al. 2001; Royal College of Paediatrics and Child Health 2002; Allen 2007; Esposito et al. 2009, 2010a; Ruggiero et al. 2011; Kersun et al. 2013a).
Multiple small studies have analyzed response to influenza vaccination, mainly during maintenance of ALL therapy and reviewed by Esposito et al. (2009) and Kersun et al. (2013a) (Allison et al. 1977; Sumaya et al. 1977; Ganz et al. 1978; Gross et al. 1978; Smithson et al. 1978; Lange et al. 1979; Schafer et al. 1979; Steinherz et al. 1980; Brydak et al. 1996, 1998; Chisholm et al. 2001; Porter et al. 2004; Matsuzaki et al. 2005; Bektas et al. 2007; Shahgholi et al. 2010; Wong-Chew et al. 2012; Kersun et al. 2013a). In general, the vaccine was well tolerated with no serious adverse side effects. When comparing response for patients receiving chemotherapy versus patients off therapy and healthy controls, rate of seroconversion was lower in patients still receiving therapy. Additionally, response in patients with solid tumors was more similar to patients off therapy and healthy controls. Kersun et al. (2013b) showed that ALL patients vaccinated during induction had an improved response compared to patients receiving the vaccine post-induction or in maintenance. Timing of immunization during an ALL maintenance cycle has not been studied to determine if the rate of response is improved when the vaccine is given separated from a 5-day steroid pulse. Additionally, patients are recommended to receive a two-shot series their first year of immunization and subsequently one annual shot. It is unclear if there would be additional benefit by continuing with a yearly two-shot series or increased dose while immunocompromised.
16.7.5.2 2009 H1N1 Pandemic Vaccine
Seven studies have reported on efficacy of the 2009 H1N1 pandemic influenza vaccine (Bate et al. 2010b; Cheng et al. 2011; Yen et al. 2011; Hakim et al. 2012; Shahin et al. 2012; Leahy et al. 2013; Mavinkurve-Groothuis et al. 2013). In general, response rates were increased after two doses of vaccine in those patients with solid tumors and in those not receiving treatment. For a mixed pediatric oncology cohort, seroresponse ranged from 25.6–100 % (Bate et al. 2010b; Cheng et al. 2011; Yen et al. 2011; Hakim et al. 2012; Leahy et al. 2013; Mavinkurve-Groothuis et al. 2013). Absolute lymphocyte counts greater than the upper limit of normal for age (or ≥1.0–1.5 x 109/L depending on the study) were a significant factor in antibody response in three studies (Yen et al. 2011; Hakim et al. 2012; Mavinkurve-Groothuis et al. 2013). Leahy et al. (2013) showed significantly improved seropositivity in children who received the higher 0.5 mL dose on univariate but not multivariate analysis. No severe adverse reactions were noted in any of the studies. As with the annual trivalent influenza vaccine, clear data as to the most efficacious timing of immunization during ALL therapy, appropriate dose and the need for one versus two doses of vaccine are lacking although repeated, higher doses appear most effective.
16.7.5.3 Live Attenuated Influenza Vaccine
Two studies have been completed to measure seroresponse and safety of the live attenuated influenza vaccine (LAIV) in immunocompromised patients (Carr et al. 2011; Halasa et al. 2011). Halasa et al. (2011) conducted a small pilot study on the safety and immunogenicity of LAIV in mild to moderately immunocompromised children with cancer. Children with severe immunodeficiency as defined by an absolute neutrophil count (ANC) <0.5 x 109/L, concurrent high-dose steroid usage (≥2 mg/kg/day) or CD4+ T-lymphocyte percentage <15 % were excluded. The ten patients with hematologic malignancies and solid tumors who were immunized did not have any serious adverse events or an excessive period of viral shedding. Immunogenicity ranged from 33–44 % depending on the assay utilized. Carr et al. (2011) compared seroresponse in 52 children who were mild to moderately immunocompromised and randomly assigned to LAIV or inactivated vaccine. Seroprotection was found to be greater to influenza A strains with the inactivated vaccine. No difference was seen in seroprotection to influenza B. No serious adverse events were noted; specifically, viral shedding was not increased with the live attenuated vaccine. With limited safety data and no evidence of increased immunogenicity with LAIV, this form of the influenza vaccine remains relatively contraindicated in pediatric oncology patients.
16.7.6 Hepatitis B Virus Vaccine
In areas of high prevalence, especially East Asia and lower-income countries, the risk of HBV transmission during chemotherapeutic regimens is significant and therefore vaccination in these settings should be strongly considered (Somjee et al. 1999; Meral et al. 2000; Sevinir et al. 2003; Yetgin et al. 2007). Multiple studies using different vaccination schedules, a combination of passive and active immunization, and significant difference in transmission risk are present in the literature (Berberoğlu et al. 1995; Hovi et al. 1995; Kavakli et al. 1996; Goyal et al. 1998; Somjee et al. 1999; Meral et al. 2000; Yetgin et al. 2001; Somjee et al. 2002; Köksal et al. 2007; Yetgin et al. 2007; Baytan et al. 2008). The lowest rate of HBV transmission appears to be in those patients that receive a combination of passive and active immunization although the cost-effectiveness of this approach is questionable (Kavakli et al. 1996; Meral et al. 2000; Somjee et al. 2002). For seronegative patients with ALL in maintenance, seroconversion rates ranged from 35.1–62.5 % after a 2–5 shot HBV series (Yetgin et al. 2001, 2007; Baytan et al. 2008). Although HBV transmission still occurs in those that are immunized during therapy, Yetgin et al. (2007) showed that infection was significantly decreased compared to unvaccinated patients, even if seroconversion did not occur. Additional studies among pediatric oncology patients with variable timing of immunization and vaccination schedules showed a seroconversion rate of 50–78 % (Berberoğlu et al. 1995; Hovi et al. 1995; Köksal et al. 2007). Hovi et al. (1995) studied 165 pediatric oncology patients; of the 51 on therapy, 67 % responded to a three-dose immunization schedule as compared to a 97 % seroresponse in the 114 off therapy. HBV immunization is important in settings outside of the United States and Western Europe where risk of transmission during therapy is high, although firm recommendations on the optimal timing and schedule of vaccination cannot be made based on the studies to date.
16.7.7 Meningococcal Conjugate Vaccine
The risk of meningococcus in immunocompromised pediatric oncology patients is unknown. Yu et al. (2007) studied vaccine response to protein-conjugated meningococcal C vaccine in 25 children with ALL and found improved response in those vaccinated 3 months after the completion of chemotherapy as compared to those in maintenance therapy (4 of 15 responders in maintenance versus 9 of 10 responders after chemotherapy completion).
16.7.8 Varicella Zoster Virus
Due to the herd immunity provided by routine varicella vaccination, especially in North America, guidelines for immunization during ALL maintenance have changed, and immunization is no longer recommended in these settings (Centers for Disease Control and Prevention 2007; American Academy of Pediatrics 2012c; Caniza et al. 2012). However, varicella immunization should still be a consideration in higher-risk populations, especially lower-income countries and, potentially, those higher income countries without universal varicella vaccination campaigns (Levin 2008; Esposito et al. 2010a).
Sartori (2004) provides an excellent review of varicella vaccination in immunocompromised patients. Early studies of the efficacy and safety of live attenuated varicella vaccine in children with acute leukemia were done in Japan with further safety data in the United States (Gershon et al. 1984; Takahashi et al. 1985; Gershon et al. 1986; Gershon and Steinberg 1989).
In their initial study, Gershon et al. (1984) showed the safety of live attenuated varicella vaccination when given to children with ALL that were in continuous clinical remission for 1 year, had a lymphocyte count ≥0.7 x 109/L, an IgG level ≥100 mg/dL, responded to at least one mitogen, and had all chemotherapy suspended for 1 week before and after vaccination. Seroresponse was 80 % after one dose of VZV. Rash was the only side effect which also increased seroconversion as well as the chance of transmission. Vaccination was quite protective, decreasing rate of infection after exposure to 18 % from an expected 90 % and also presenting as mild disease in those with clinical illness after exposure. A follow-up study by the same group with a larger cohort showed 88 % seroconversion after one dose of vaccination and 98 % seroconversion after two doses with no notable serious adverse events (Gershon and Steinberg 1989). Multiple other small studies have shown similar results with no serious adverse events (Heath and Malpas 1985; Ninane et al. 1985; Heath et al. 1987; Ecevit et al. 1996; Cakir et al. 2012).
A recent case report by Schrauder et al. (2007) on a child with ALL who developed fulminant varicella infection 32 days after varicella vaccination deserves mention. Vaccination was given with an interruption in chemotherapy, 1 week prior and 1 week after, but was given 5 months after complete remission had been achieved and prior to intensive reinduction chemotherapy, not in accordance with the stringent guidelines set forth by Takahashi et al. (1985) and Gershon et al. (1984, 1986; Gershon and Steinberg 1989). Based on their case, the authors recommend waiting at least 9 months after all therapy completion (including maintenance chemotherapy) prior to administering varicella vaccination. Their recommendation is not supported by the existing literature but does emphasize the care and attention that is necessary when administering this live attenuated vaccine to children that remain immunocompromised (Centers for Disease Control and Prevention 2007).
16.8 Recommendations for Vaccination After Chemotherapy Completion
Immune reconstitution is variable after the completion of chemotherapy leading to inconsistent guidelines for (re)vaccination. Among the published guidelines, four authors recommend commencing (re)vaccination 3 months after therapy completion (Centers for Disease Control and Prevention 1993; Sung et al. 2001; Allen 2007; Esposito et al. 2010a). For three of the four authors, this recommendation is inclusive of live viral vaccines (Centers for Disease Control and Prevention 2007). Esposito et al. (2010a) recommend waiting 6 months for live vaccines. The UK guidelines (Royal College of Paediatrics and Child Health 2002) recommend waiting 6 months for all vaccinations while Ruggiero et al. (2011) recommend waiting 6 months for inactivated/killed vaccines and measles but 12 months for VZV. Fioredda et al. (2005) also recommend waiting 6 months after therapy completion. Guidelines are unclear in regard to children that interrupted their primary immunization series (Esposito et al. 2010a; Ruggiero et al. 2011). Based on their review, van Tilburg et al. (2012) conclude that although revaccination is important, further study is still required to determine what the appropriate immunizations are, depending on the local herd immunity and risk for vaccine-preventable disease after chemotherapy. They do feel based on their review that 3 months after the completion of therapy is a good time point to begin the evaluatory process. Whether the evaluatory process should include pre- and/or post-immunization titers is also unclear; additionally there is a significant associated cost with such a strategy and seroprotection may not always equate with seropositivity by antibody level. The main factors that must be considered are minimizing the period of risk to the patient while balancing the risk for lack of seroconversion with premature immunization. Risk of vaccine-related infection from live viral vaccination seems less of a concern after therapy completion since patients can safely be immunized with varicella during ALL maintenance. For influenza, Esposito et al. (2010b) show that the biggest risk to pediatric patients with malignancy is during treatment and 6 months after the completion of therapy (including risk of infection and hospitalization). Beyond this point, risk becomes similar to the general pediatric population.
Based on the UK guidelines as outlined in the Royal College of Paediatrics and Child Health (RCPCH) best practice statement from 2002, Patel et al. (2007) enrolled 59 children with a history of leukemia ≥6 months after chemotherapy completion for revaccination. They found the large majority who were deficient achieved optimal antibody concentrations that persisted when rechecked 12 months after immunization. Based on their results they recommend following the RCPCH timing for booster vaccination. Of their studied vaccinations, inactivated poliovirus vaccine was the least immunogenic (HBV was not part of the study) but seroconversion rates were similar to published response in healthy individuals. Treatment intensity was not significantly associated with seroresponse. Similar rates of seroconversion are plausible with earlier vaccination as well; thus, 3 months post the completion of therapy is recommended by several authors based on their data (Lehrnbecher et al. 2009; Zengin and Sarper 2009). Large, randomized controlled trial data are lacking to make firm recommendations.
16.9 Active/Passive Immunization After Disease Exposure
16.9.1 Varicella
Live virus vaccination is contraindicated after varicella disease exposure (2 days prior to rash or before all lesions crusted over in contact) in immunocompromised individuals, although passive immunization and antivirals may be of utility. Multiple studies of variable quality have shown the potential benefit of varicella zoster immune globulin (VariZIG; VZIG) in immunocompromised children, nicely summarized by Fisher et al. (2011) (Brunell et al. 1972; Gershon et al. 1974; Judelsohn et al. 1974; Feldman et al. 1975; Evans et al. 1980; Orenstein et al. 1981; Hanngren et al. 1983; Zaia et al. 1983; Feldman and Lott 1987). VZIG often will not prevent disease in immunocompromised patients but has been shown to decrease disease severity (in most patients). Efficacy of VZIG has been shown to decline if given >72 h after exposure; therefore, previous US recommendations were to administer it within 96 h of exposure (Feldman and Lott 1988; American Academy of Pediatrics 2006). With the potential to attenuate disease even beyond this 72–96 h window, newer US guidelines as well as UK guidelines recommend VZIG up to 10 days after exposure (Royal College of Paediatrics and Child Health 2002; American Academy of Pediatrics 2012c). Due to the lack of quality studies, VariZIG remains an investigational agent in the United States and requires institutional review board approval and completion of an investigational new drug form. If VZIG is not available, intravenous immunoglobulin (IVIG) may be given.
Oral acyclovir antiviral prophylaxis has been claimed to show benefit in multiple studies, summarized by Fisher et al. (2011) (Ishida et al. 1996; Goldstein et al. 2000; Martin-Hernandez 2000; Shinjoh and Takahashi 2009). As with VZIG, these studies are case reports or nonrandomized uncontrolled studies. Studies in healthy children have specifically shown a decrease in disease when acyclovir is given as a 7-day course starting 1 week after exposure; efficacy was decreased when prophylaxis was started 3 or 11 days after exposure (Asano et al. 1993; Suga et al. 1993; Huang et al. 1995; Fisher et al. 2011). Current US guidelines recommend a 7-day course starting 7–10 days after exposure, while UK guidelines recommend a 14-day course starting 7 days after exposure (Royal College of Paediatrics and Child Health 2002; American Academy of Pediatrics 2012c). See Table 16.2 for VZIG, IVIG and acyclovir dosing recommendations. Fisher et al. (2011) comment in their review that a formal comparison between VZIG and acyclovir is lacking.
Table 16.2
Passive immunization after varicella or measles disease exposurea
Exposure to varicella 2 days prior to rash or before crusting of all lesions in contact: |
If within 4–10 days of exposure : |
VZIG 125 units/10 kg for the first 10–40 kg; >40 kg, 625 units IM (max 2.5 mL per injection site) |
Or |
IVIG 400 mg/kg IV |
Or |
If within 7–10 days of exposure and neither VZIG nor IVIG administered : |
Acyclovir 80 mg/kg/day PO div QID (max dose 800 mg QID), for 7–14 days |
Exposure to measles 5 days prior to or 4 days after onset of rash in contact: |
If within 6–14 days of exposure : |
Immunoglobulin 0.5 mL/kg IM (max dose 15 mL; max 3 mL per injection site in children) |
Or |
IVIG 400 mg/kg IV |
16.9.2 Measles
Measles immunization is contraindicated after measles exposure in immunocompromised patients. Passive immunization with immunoglobulin (Ig) should be utilized especially with virologic confirmation of exposure and exposure occurring 5 days prior to and up to 4 days after the onset of rash in the infectious contact. Ig may be given either intramuscularly or intravenously, especially if thrombocytopenic. Ideally passive prophylaxis should be given within 72 h of exposure; US guidelines recommend Ig up to 6 days after exposure, UK guidelines up to 14 days after contact (Royal College of Paediatrics and Child Health 2002; American Academy of Pediatrics 2012b). See Table 16.2 for dosing. Of note, a washout period after any immunoglobulin product (and blood products) is required prior to administration of measles vaccination. In the previously immunocompromised patient, MMR should be given a minimum of 6 months after Ig (American Academy of Pediatrics 2012b). In settings without Ig availability, early initiation of ribavirin for the treatment or postexposure prophylaxis of measles can be considered (Moulik et al. 2013).
16.10 Treatment of Hypogammaglobulinemia During Chemotherapy
The impact of low immunoglobulin levels on the risk of infectious sequelae during chemotherapy has not been well characterized. Although van Tilburg et al. (2012) showed that IgG levels were significantly lower in ALL patients receiving more intensive therapy and these patients also suffered more infectious complications, this fact could not be directly correlated to IgG levels. Kovacs et al. (2008) analyzed 88 children 1 year after the completion of chemotherapeutic regimens for malignancies. Leukemia patients suffered a statistically increased number of febrile episodes as compared to solid tumor patients, although this did not correlate with immunoglobulin levels. Similarly, solid tumor patients with low immunoglobulin levels suffered more febrile episodes than those with normal Ig levels, but not to the point of statistical significance. Multiple consensus statements on the use of IVIG do not include routine use in acquired hypogammaglobulinemia due to chemotherapy (Hemming 2001; Orange et al. 2006; Robinson et al. 2007). In a Canadian consensus statement, Robinson et al. (2007) note that IVIG is often a part of oncologic study protocols (though not evidence-based) and may also be considered in patients with a history of severe invasive infection or recurrent sinopulmonary infection in the setting of acquired hypogammaglobulinemia.
16.11 Vaccination of Household Contacts
Minimizing the risk of exposure in immunocompromised patients to vaccine-preventable diseases by immunization of household contacts is a vital aspect of supportive care (Table 16.3). As discussed, immunogenicity to vaccine-preventable disease will be blunted during the period of highest risk; thus, minimizing any potential infectious contacts is more important than vaccine guidelines in those receiving therapy. Immunization of healthcare workers is therefore also important and summarized in Chap. 14. Live virus vaccines including measles-mumps-rubella, rotavirus and varicella have all been deemed safe due to the minimal risk of disease spread. Oral poliovirus vaccine is contraindicated and live attenuated influenza vaccine is relatively contraindicated (American Academy of Pediatrics 2012a). Household contacts should receive yearly inactivated influenza vaccine and young, susceptible contacts should be immunized against varicella. Vaccinees who develop a postvaccination rash should be separated from susceptible individuals due to the theoretical risk of infection transmission (Hughes et al. 1994; LaRussa et al. 1997; Chaves et al. 2008; Galea et al. 2008). However, no transmission of vaccine strain varicella has been reported to immunocompromised patients in the United States after 55 million doses of vaccine have been given (Chaves et al. 2008; Galea et al. 2008). Outside of the United States, in countries without national varicella vaccination programs, immunization of household contacts has been problematic due to concerns of safety as well as a lack of identification by pediatric oncologists (Timitilli et al. 2008; Fisher et al. 2011).
Table 16.3




Vaccination recommendations in household contacts of immunocompromised patientsa
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree