Chapter 13 Immunity to Viruses
• Innate immune responses (mediated by anti-microbial peptides, type I interferons (IFNs), dendritic cells (DCs), natural killer (NK) cells, and macrophages) restrict the early stages of infection, delay spread of virus and promote the activation of adaptive responses. Innate defences are triggered following recognition of molecular ‘patterns’ characteristic of viral but not host components. Type I IFNs exert direct antiviral activity and also activate other innate and adaptive responses. NK cells are cytotoxic for virally infected cells. Macrophages act at three levels to destroy virus and virus-infected cells.
• As a viral infection proceeds, the adaptive (specific) immune response unfolds. Antibodies and complement can limit viral spread or reinfection. T cells mediate viral immunity in several ways – CD8+ T cells destroy virus-infected cells or cure them of infection; CD4+ T cells promote antibody and CD8+ T cell responses and are a major effector cell population in the response to some virus infections.
• Viruses have evolved strategies to evade the immune response. They may impair the host immune response at the induction and/or effector stages; avoid recognition by the immune response, e.g. via latency or antigenic variation; or resist control by immune effector mechanisms. Many viruses employ multiple strategies to prolong their replication in the host.
• Responses induced during viral infections can have pathological consequences. Damage can be mediated by antiviral responses (e.g. via the formation of immune complexes or T cell-induced damage to host tissues) or by autoimmune responses triggered during the course of infection.
Innate immune defenses against viruses
Type I interferons have critical antiviral and immunostimulatory roles
• type I (multiple subtypes of IFNα and one subtype of IFNβ);
• type III (IFNλ1, IFNλ2, and IFNλ3, also known as IL-29, IL-28a, and IL-28b).
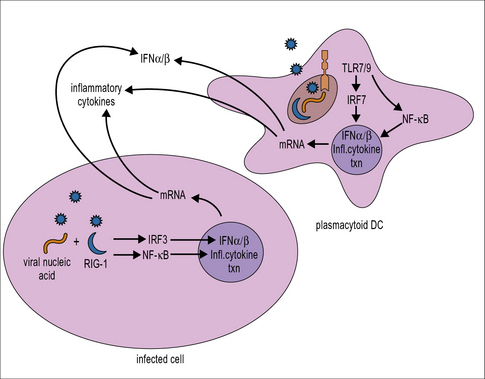
Type I IFN production is triggered following recognition of molecular patterns characteristic of viral but not host components (Fig. 13.1). Host pattern-recognition receptors involved in detecting the presence of virus infections include:
• cytoplasmic pattern-recognition receptors expressed by almost all cells (e.g. the retinoic acid-inducible gene I (RIG-I)-like receptors, which recognize viral 5′-triphosphorylated ssRNA and dsRNA, and cytoplasmic DNA sensors);
• members of the Toll-like family of receptors (TLRs), which are expressed on the cell membrane or within endosomes/lysosomes of immune system cells and certain non-immune cells located at common sites of pathogen entry, e.g. epithelial cells (TLR3, TLR7, and TLR9, which recognize viral dsRNA, viral ssRNA, and DNA containing CpG motifs, respectively).
Triggering of pattern-recognition receptors initiates signaling along pathways that culminate in the activation of transcription factors including IFN regulatory factor (IRF)3 and NFκB, which translocate into the nucleus and activate the transcription of type I IFNs and inflammatory cytokines, respectively (see Fig. 13.1). Plasmacytoid DCs also have a unique signaling pathway for induction of type I IFN production in response to TLR7 or TLR9 ligation that involves the transcription factor IRF7.
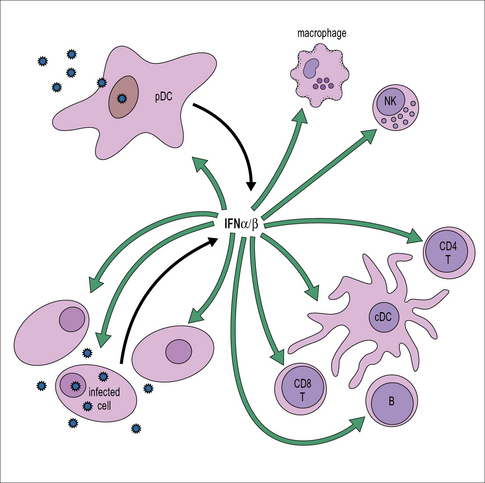
The IFN released acts on both the cell producing it and also neighboring cells where it establishes an antiviral state, enabling them to resist virus infection (Fig. 13.2).
• PKR disrupts virus infection by phosphorylating and inhibiting eukaryotic initiation factor (eIF)-2α, hence blocking the translation of viral mRNA and by initiating apoptosis via Bcl-2 and caspase-dependent mechanisms, killing the cell before virus can be released.
• 2′,5′-oligoadenylate synthetase specifically activates a latent endonuclease (RNaseL) that targets the degradation of viral RNA.
In addition to the direct inhibition of virus replication, IFNs also activate macrophages and NK cells and enhance their antiviral activity (see Fig. 13.2). In addition, they help to promote the activation of adaptive responses. They act on antigen presenting cells including conventional DCs to stimulate increased expression of MHC class I and II, along with components of the antigen processing machinery; and they also act directly on T and B cells to promote an antiviral response (see Fig. 13.2).
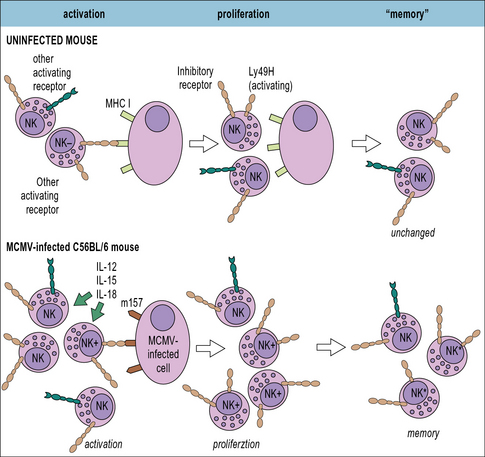
NK cells are cytotoxic for virally-infected cells
• Inhibitory NK receptors typically recognize ligands expressed on ‘normal’ host cells, such as MHC class I molecules. As discussed below, many viruses down-regulate MHC class I expression on the cells they infect to limit recognition by CD8+ T cells, but this helps to trigger NK cell activation.
• Activating NK receptors typically recognize host cell proteins that are up-regulated in response to stress or viral proteins, e.g. the natural cytotoxicity receptors NKp44 and NKp46 recognize certain viral glycoproteins including the influenza virus HA. Mice deficient in NKp46 are highly susceptible to influenza virus infection.
• NK cells can also be activated via antibody coating of the target cell which mediates crosslinking of the NK surface receptor FcγRIII. As discussed below, NK cells are one of the principal mediators of antibody-dependent cell-mediated cytotoxicity (ADCC).![]()
Adaptive immune responses to viral infection
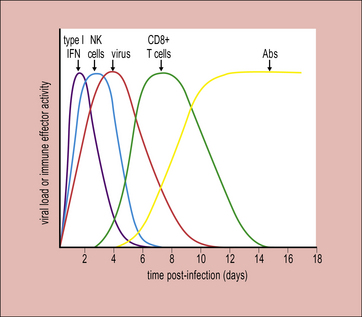
The adaptive immune response typically begins a few days after innate responses are activated (Fig. 13.3). T cells start to appear at sites of infection around 4 days after the initiation of viral expansion. In many virus infections, it is the action of CD8+ T cells that plays a key role in the resolution of infection. Antibodies are frequently induced slightly later, around day 6/7, and contribute to recovery from infection.