Chapter 22 Immunity to Cancers
• The immune system can potentially survey the body for some types of developing tumor.
• Tumors can induce immunity. Mice, rats, hamsters, and frogs can be immunized against tumors. In most tumors of animals, tumor immunity elicited by immunization is specific (or strongest) to the individual tumor that was used to immunize.
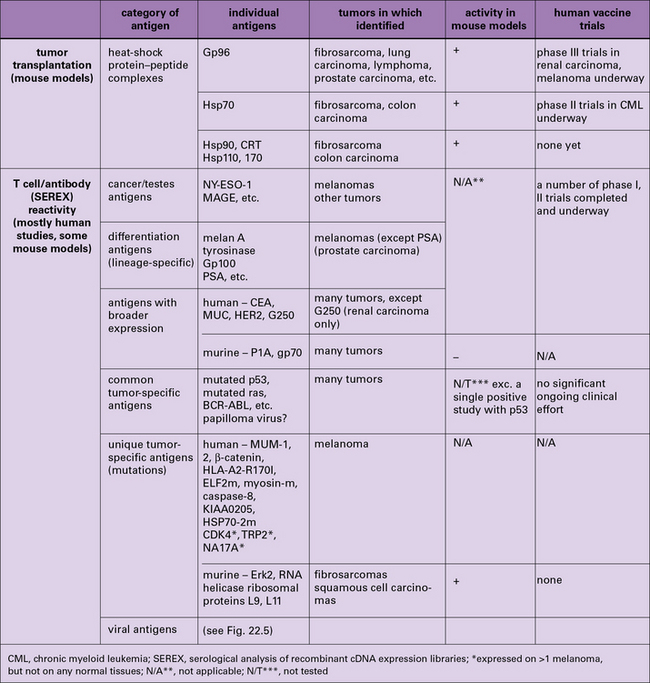
• Tumor antigens have been characterized by three means – immunization-challenge experiments, T cell reactivity, and antibody reactivity. Immunization-challenge experiments have uncovered the immunogenicity of heat-shock protein-chaperoned antigenic peptides. Antigens identified by reactivity to T cells and antibodies include individual tumor-specific mutated antigens, cancer testes antigens, differentiation antigens, and viral tumor antigens.
• Vigorous anti-tumor immune responses are compromised by regulatory mechanisms. Tumors elicit immunity in their primary host, and such immunity is downregulated. Regulatory cells such as CD25+ CD4+ T cells and inhibition of activated anti-tumor T cells through T cell molecules such as CTLA-4 are involved in downregulation of tumor immunity.
Tumor immunity in the primary host
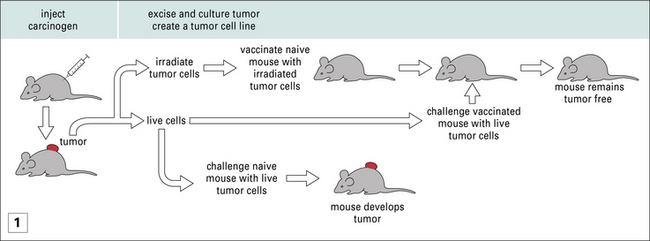
Nonetheless, amidst the barrage of experiments where MHC-mismatched tumors were transplanted into mice, were the experiments of Ludwik Gross, and later those of Prehn and Main, and of George and Eva Klein, who showed that, even when MHC-matched tumors were used to immunize mice, protection against subsequent tumor growth could be achieved (Fig. 22.1). These studies led to two principles, which have informed much of cancer immunology since and are discussed below.
Cancers elicit protective immunity in the primary and syngeneic host
• The immunogenicity of tumors has provided the foundation stone for the idea of tumor-specific antigens. If one could immunize, then antigens must exist.
• Tumor immunity depends on many factors. The degree of tumor immunity depends upon the type of cancer and the method of its induction, or lack of induction. UV-induced cancers are highly immunogenic, methyl-cholanthrene-induced tumors less so, and spontaneous tumors even less so. Nonetheless, immunogenicity of tumors has been demonstrated in all model systems tested.
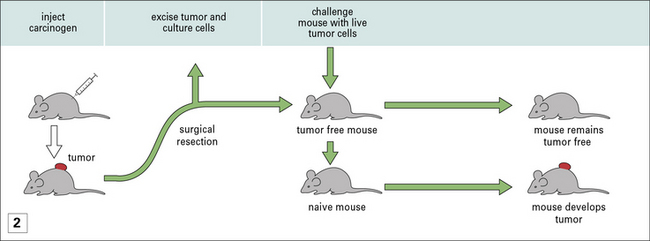
• The primary animal develops immunity to subsequent tumor challenge. Furthermore it is also immune to subsequent challenges with the same tumor (see Fig. 22.1).
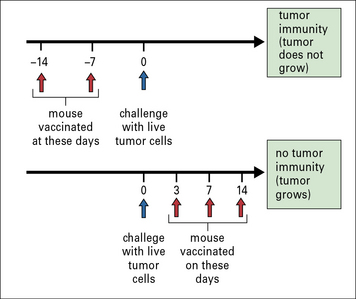
• Protective immunity is only seen in prophylactic immunization and not therapeutic immunization – once a mouse has been implanted with a tumor, immunization with irradiated cancer cells (derived from the growing tumor) does nothing to mitigate tumor growth (Fig. 22.2). Exploration of differences between prophylaxis and therapy has led to fundamental insights into tumor immunity.
Spontaneous tumors are also antigenically distinct
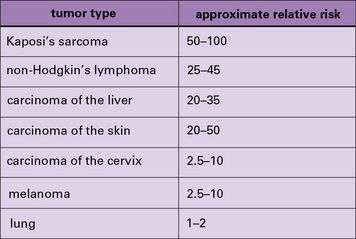
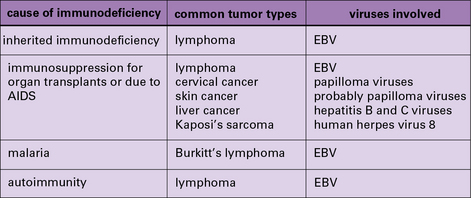
In addition to these experimental studies, several clinical observations point to the existence of tumor-protective immunity in humans. These include the increased relative risk of cancers in patients who are immunosuppressed because they are kidney transplant recipients (Fig. 22.3) or for a variety of other reasons (Fig. 22.4).
Characterization of tumor antigens
The two broad approaches to the identification of tumor antigens are shown in Figure 22.5 and discussed individually below. Not surprisingly, the approaches have yielded results that are not fully concordant. These differences have helped highlight a fascinating interplay between immunity and tolerance to tumors, discussed below.
Tumor-specific antigens defined by immunization all belong to the family of HSPs
• could elicit protective immunity;
• were of the HSP90 (gp96 and HSP90), HSP70 (HSP110 and HSP/c70), calreticulin, and HSP170 (also known as grp170) families.
HSPs must be isolated directly from tumors to be immunologically active
Two aspects of HSP-elicited tumor immunity are notable.
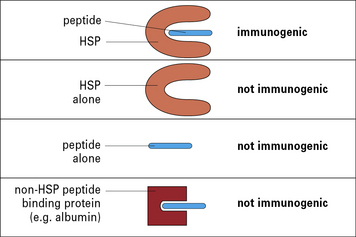
• First, HSPs are present in normal tissues as well as tumors, and normal tissue-derived HSPs do not elicit rejection. They must be isolated from tumors to be immunologically active.
• Second, HSPs elicit immunity specifically against the individual tumors from which they are isolated.
This conundrum was resolved by the demonstration that the HSP molecules chaperone peptides in a peptide-binding pocket, much as the MHC molecules do, although the structural details of the pockets in HSP and MHC differ (Fig. 22.6). The specificity of immunogenicity derives from the peptides rather than the HSP itself – dissociation of HSP-associated peptides from HSPs abrogate the tumor rejection activity.
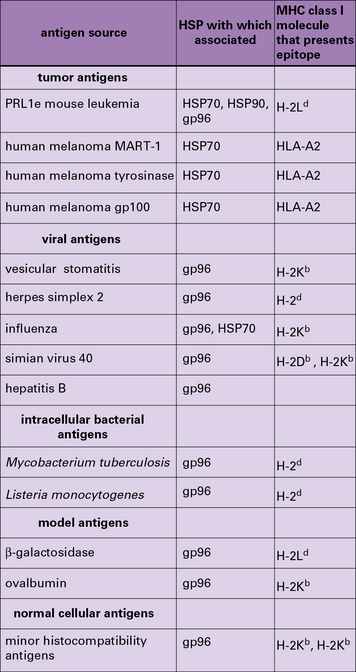
HSPs can chaperone many different types of antigen
HSP-chaperoned peptide pools contain cytotoxic T lymphocyte (CTL) epitopes and CTL epitope precursors for any antigens that the cell expresses (Fig. 22.w1). This evidence comes from mouse and human tumors, normal tissues, and virus-infected cells.
HSP molecules bind to APCs and target peptides with high efficiency
The HSP molecule itself plays at least two crucial roles other than chaperoning peptides:
• HSPs bind antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) through HSP receptors such as CD91 and thus target the peptides chaperoned by them into the APCs with high efficiency;
• further, the HSP-chaperoned peptides, once introduced into the APC, follow the endogenous as well as the exogenous pathway of antigen presentation and are processed and re-presented by the MHC I and MHC II molecules of the APCs (Fig. 22.7).
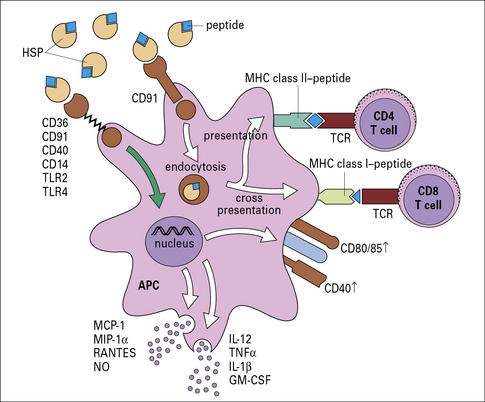
Fig. 22.7 Mechanism of specific immunogenicity of HSP-peptide complexes
(Redrawn from Srivastava P. Nat Rev Immunol 2002;2:185–194. Copyright 2002, Nature Reviews Immunology.)