Chapter 12 Immune Responses in Tissues
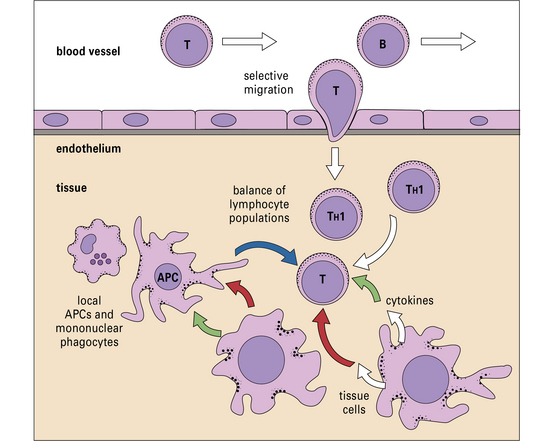
• A tissue can influence local immune responses, promoting some classes of immunity and suppressing others. Vascular endothelium in each tissue expresses chemokines and adhesion molecules that attract specific subsets of leukocytes.
• Certain sites in the body are immunologically privileged and fully allogeneic tissue can be transplanted into them without risk of rejection. These sites, which include the anterior chamber of the eye and the CNS, promote beneficial classes of immune responses while suppressing classes that can do irreparable local damage.
• The endothelium in the CNS has barrier properties, which exclude most serum proteins from the tissue. Acute inflammation in the CNS is characterized by TH1 cells, TH17 cells and mononuclear phagocytes.
• Immune responses in gut and lung distinguish between pathogens and innocuous organisms and antigens. The immune response in mucosal tissues tends to promote TH2-type responses with IgA production. Gut enterocytes influence the local immune response. Intraepithelial lymphocytes (IELs) respond to stress-induced class Ib molecules and produce many immunomodulatory cytokines. Regulatory T cells normally limit the level of inflammatory reactions.
• T cells are present in normal skin and immune responses are characterized by T-cell infiltration. The endothelium of the dermis attracts TH1 cells, which express cutaneous lymphocyte antigen (CLA) and receptors for IFNγ-induced chemokines.
Tissue-specific immune responses
What determines whether an immune response should be comprised of, for example, activated cytotoxic T lymphocytes (CTLs) or a particular class of antibodies? Although immune responses are primarily tailored to the pathogen, there is also a strong influence from the local tissue, where the immune response occurs (Fig. 12.1).
• the features of immune responses that are unique to individual tissues; and
• the mechanisms by which the tissues influence local and systemic characteristics.
There are several reasons why a particular organ may need to modify local immunity.
Moreover, some types of immune response are only appropriate in specific tissues.
Locally produced cytokine and chemokines influence tissue-specific immune responses
• a tissue can promote some classes of immunity and suppress others;
• the vascular endothelium plays a major role in determining which leukocytes will enter the tissue by secretion of distinct blends of chemokines, and expression of site specific adhesion molecules;
• cells in the tissue can exert their effects via cytokines or by direct cell–cell interactions. In effect, cells of the tissue can signal infection, damage or distress;
• a tissue can have several microenvironments, each of which has its own physiology and preferred immune response;
Endothelium controls which leukocytes enter a tissue
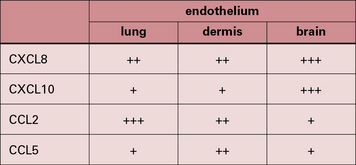
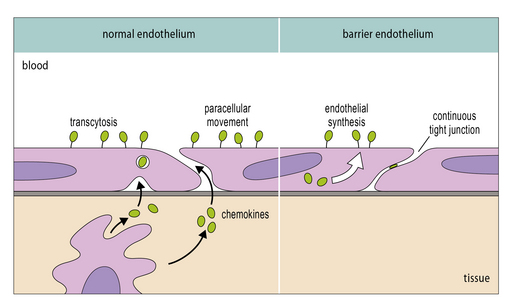
It is now clear that a major element controlling inflammation and the immune response is the vascular endothelium in each tissue, which has its own characteristics; different endothelia produce distinctive blends of chemokines (Fig. 12.2). In addition the endothelium can transport chemokines produced by cells in the tissue from the basal to the lumenal surface by transcytosis, or by surface diffusion in tissues that lack barrier properties (Fig. 12.3).
Hence the different sets of leukocytes present in each tissue can be partly related to the chemokines synthesized by the cells present, particularly the endothelium. For example, in normal lung there is a high level of macrophage migration, which relates to the high expression of CCL2 (macrophage chemotactic protein-1) by lung endothelium. In allergic asthma, the proportion of eosinophils increases, due to the production of IL-5 and CCL3 (eotaxin), which are characteristic of the TH2 response that tends to predominate in mucosal tissues (Fig. 12.4). By contrast, in the CNS lymphocytes and mononuclear phagocytes predominate in most immune reactions.
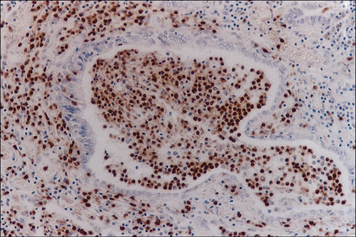
Fig. 12.4 Histological section of an airway from a case of fatal asthma
(Courtesy of Arshad SH. Allergy: An Illustrated Colour Text. Philadelphia: Churchill Livingstone, 2002. With permission from Elsevier.)
Immune reactions in the CNS
• the blood–brain barrier (endothelium plus astrocytes) prevents the movement of over 99% of large serum proteins into the brain tissue (IgG, complement, etc.); there are similar barriers in the eye (blood–retinal barrier);
• low levels of MHC molecule expression, and co-stimulatory molecules result in inefficient antigen presentation;
• there is no conventional lymphatic drainage system from brain tissue to local lymph nodes;
• there are low levels of leukocyte traffic into the CNS in comparison with other tissues;
• neurons have direct immunosuppressive actions on glial cells;
• astrocytes, neurons and some glial cells produce immunosuppressive cytokines.
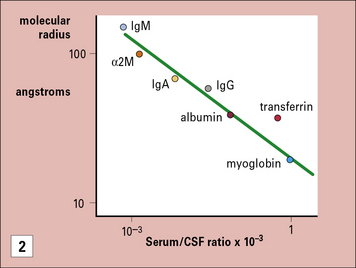
The blood–brain barrier excludes most antibodies from the CNS
The blood–brain barrier is a composite structure formed by the specialized brain endothelium and the foot processes of astrocytes. Astrocytes are required to induce the special properties of brain endothelial cells, which have continuous belts of tight junctions connecting them to other endothelial cells (Fig. 12.5). An estimate of the tightness of the barrier is given by its trans-endothelial resistance, which is up to 2000 Ω/cm2 in the CNS by comparison with values <10 Ω/cm2 in most other tissues. In addition the brain endothelial cells have an array of transporters that allow nutrients into the CNS and a set of multi-drug resistance pumps that prevent many toxic molecules and therapeutic drugs from entering the brain. However, it is the very low permeability of the endothelium to serum proteins which is of particular interest for immunologists (see Fig. 12.5). For example, the level of IgG found in the CNS is normally approximately 0.2% of the level found in serum. The level may rise during an immune reaction as the endothelial barrier becomes more permeable in response to inflammatory cytokines. In some conditions, such as multiple sclerosis, there is often local synthesis of antibody within the CNS, which is reflected in abnormally high antibody levels in cerebrospinal fluid, even accounting for the increased leakage into the CNS. This finding demonstrates that some B cells have migrated into the CNS, and plasma cells have been identified in the spaces surrounding the larger blood vessels. Macrophages also contribute to immune reactions in CNS and they can synthesize some complement components locally (e.g. C3). However the overall level of serum proteins including antibodies and complement rarely exceeds 2% of the levels in serum even in the most severe inflammatory reactions.