- 7.1 Introduction
- 7.2 Immunosuppression
- 7.2.1 Drugs
- 7.2.2 Polyclonal antibodies for prevention of responses
- 7.2.3 Non-specific immunomodulation by intravenous immunoglobulin
- 7.2.4 Monoclonal antibodies for specific immunomodulation
- 7.2.5 Other uses of monoclonal antibodies
- 7.2.6 Fusion proteins for blocking receptors
- 7.2.7 Plasmapheresis and plasma exchange
- 7.2.8 Total lymphoid irradiation
- 7.2.9 Ultraviolet light
- 7.2.1 Drugs
- 7.3 Immunization against infection
- 7.3.1 Theoretical basis of immunization
- 7.3.2 Adjuvants
- 7.3.3 Routine immunization
- 7.3.4 Immunization for travellers
- 7.3.5 Passive immunization
- 7.3.1 Theoretical basis of immunization
- 7.4 Immune potentiation other than vaccines
- 7.4.1 Cytokine therapy
- 7.4.2 Gene therapy
- 7.4.3 Cancer immunotherapy
- 7.4.1 Cytokine therapy
 Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
7.1 Introduction
Although the immune system usually responds appropriately to foreign antigens, there are patients whose disease is caused by immune responses that are excessive or defective. The aim of clinical immunology is to correct these abnormalities. Two major approaches are possible: immunosuppression or immunopotentiation. An overactive, self-damaging immune system requires some degree of immunosuppression; this is the mainstay of the management of organ transplantation and certain life-threatening autoimmune diseases. With the major exceptions of immunization and human stem cell transplantation [HSCT], immunopotentiation to improve a naive or defective immune system is still unproven, although gene therapy has exciting potential. Some methods of immune manipulation produce definite clinical benefit by mechanisms which are poorly understood, e.g. therapy with intravenous immunoglobulin (IVIG) though monoclonal antibodies can suppress, potentiate or modify immune responses depending on their action, specificity and the clinical circumstances.
7.2 Immunosuppression
7.2.1 Drugs
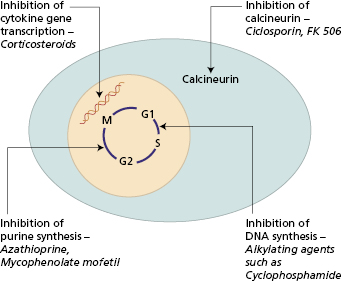
There are several groups of immunosuppressive drugs (Fig. 7.1). Their effects on the immune system are divided into short-lived changes on cell traffic and more persistent effects on individual cell functions. Their anti-inflammatory properties are separate from those on the immune system. In general, azathioprine and cyclophosphamide act on the maturation of cells, while steroids and fungal derivatives inhibit the functions of mature cells.
Fig. 7.1 Schematic depiction of the main intracellular sites of action of the major groups of immunosuppressive drugs.
Corticosteroids are primarily anti-inflammatory though they also affect cell trafficking. A single dose of corticosteroids causes changes in cell traffic within 2 h of administration; the result is a transient lymphopenia, which peaks at 4 h but is no longer apparent after 24 h. Lymphopenia occurs largely because Th cells are redistributed and Tc cells sequestered in the bone marrow; in addition, though, there is increased lymphocyte apoptosis (Table 7.1).
Table 7.1 Actions of corticosteroids on the human immune system
Inhibition of inflammation:
|
Inhibition of wound healing and repair:
|
Changes in cell traffic;
|
Th, Helper T cells; TC, cytotoxic T cells; IL, interleukin; TNF-α, tumour necrosis factor-α; IFN-γ, interferon-γ.
The influence of steroids on cell function varies according to species, dose and timing. The major effect in humans is on ‘resting’ macrophages (Table 7.1); activated macrophages are not sensitive to corticosteroids. Reduced antigen handling by macrophages probably accounts for the poor primary antibody response seen following corticosteroid administration. The secondary antibody response is not affected, as memory cells are resistant to the effects of corticosteroids.
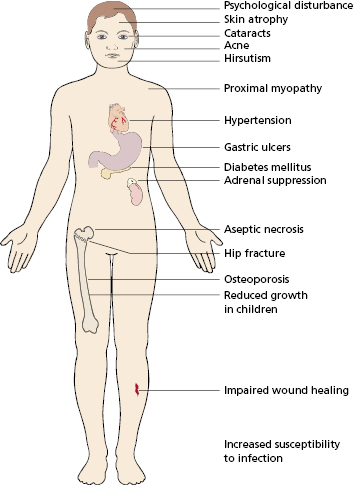
In humans, corticosteroids are used for several purposes: the prevention or reversal of graft rejection and the treatment of autoimmune, allergic and malignant diseases. In transplantation (see Chapter 8) their anti-inflammatory action and reduction of macrophage activity results in reduced cellular infiltration. Steroids alone are ineffective in preventing rejection in the early phase, although high doses (methyl prednisolone) do reverse acute rejection. Corticosteroids have a wide range of side-effects (Fig. 7.2). They are known to increase the patient’s susceptibility to infections of all kinds. Failure to export neutrophils into the tissues (Table 7.1) and reduced macrophage function is most relevant to this increased risk. Adverse effects are related to the duration of treatment as well as the dose; by giving larger doses for shorter periods, it is often possible to reduce infection risk while conserving immunosuppression. Alternatively alternate-day therapy or using steroid-sparing agents also reduces the unwanted effects.
The development of thiopurines in the 1950s provided another important immunosuppressive drug, namely azathioprine. It is inactive until metabolized by the liver and takes a few weeks to be effective. The metabolites can affect all dividing cells by inhibition of DNA synthesis (Fig. 7.1). Azathioprine is widely used in two main clinical situations: (i) prevention of rejection after organ transplantation; (ii) treatment of systemic autoimmune disease. Azathioprine affects several aspects of immune function (Table 7.2), which accounts for its bone marrow toxicity. Most patients on long-term therapy eventually show granulocytopenia and thrombocytopenia. Homozygous deficiency of thiopurine methyl transferase (TPMT), a key enzyme responsible for metabolizing azathioprine, is associated with life-threatening marrow aplasia. This homozygous deficiency is present in approximately one in 300 individuals, whereas the heterozygous state is found in 10% of the population. Measurement of TPMT enzyme activity and/or TPMT genotypes prior to initiating therapy with azathioprine, or the related compound 6-mercaptopurine, can help prevent the toxicity associated with slow metabolism. Close monitoring of leukocyte and platelet counts is critical in all patients.
Table 7.2 Actions of thiopurines on the human immune system
1 Cell traffic Acute
|
2 Effects on inflammation
|
Mycophenolate mofetil is a purine inhibitor that inhibits inosine monophosphate dehydrogenase (IMP), a key enzyme in the de novo synthesis of purines in activated T and B lymphocytes. It is an excellent substitute for azathioprine if treatment fails or there is marrow toxicity, and is now a well-established component of maintenance immunosuppressive regimens following organ transplantation.
Alkylating agents, such as cyclophosphamide, interfere with DNA duplication at the premitotic phase and are most effective in rapidly dividing cells. Tissues vary in their ability to repair DNA after alkylation, which accounts for their differing sensitivities to this group of drugs. They have little anti-inflammatory activity and so are often given with steroids. Cyclophosphamide also requires metabolism by the liver to form its active metabolites. When cyclophosphamide is given with, or immediately after, an antigen there is reduced antibody production and impaired delayed-type hypersensitivity. At low doses, CD8+ cells show a short-lived fall in number. As the dose is increased, numbers of CD4+ cells fall progressively increasing infection risk considerably. After stopping cyclophosphamide, recovery takes weeks or months as in Case 7.1 Cyclophosphamide therapy is also associated with reactivation of latent viral infectious agents such as CMV and Varicella zoster. Prolonged high doses are associated with bladder cancer, due to the carcinogenic metabolite, acrolein. Clinically, cyclophosphamide is particularly useful in aggressive autoimmune diseases (such as granulomatosis with polyangiitis or vasculitis associated with systemic lupus erythematosus), and in conditioning haematopoetic stem cell transplant [HSCT] recipients (see Chapter 8). It is also used with other antineoplastic treatments in many haematological and solid malignancies. Another alkylating agent, chlorambucil, is widely used for treating low-grade B-cell neoplasms, such as chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma. It appears to act on B cells directly. Chlorambucil is given either intermittently or in low dosage, because persistently high doses are associated with subsequent development of leukaemia.
 Case 7.1 Pneumocystis pneumonia complicating immunosuppressive therapy
Case 7.1 Pneumocystis pneumonia complicating immunosuppressive therapyA 35-year-old man with granulomatosis with polyangiitis (GPA) (formerly Wegener’s granulomatosis) was admitted to hospital with a 2-week history of fever and shortness of breath. The diagnosis of GPA had been made 18 months earlier when he presented with haemoptysis and glomerulonephritis. Disease remission was achieved with aggressive immunosuppressive therapy using a combination of pulse methylprednisolone and cyclophosphamide, enabling him to be maintained on his current tapering dose of steroids and azathioprine. The results of investigations on his current hospital admission were as follows:
- Chest X-ray: diffuse bilateral shadowing
- Serum C-reactive protein (CRP): 80 mg/l (NR < 10)
- Anti-neutrophil cytoplasmic antibody directed against proteinase 3: weakly positive at a titre of 1 : 40 (>1 : 640 at disease diagnosis)
- Serum creatinine: 102 μmol/l (NR 50–140)
- Urea: 4.5 mmol/l (NR 2.5–7.1)
- Urine microscopy: clear.
The differential diagnosis was between active GPA and infection complicating immunosuppressive therapy. It was crucial to distinguish between infection and active vasculitis in this situation, since an increase in immunosuppressive therapy in the face of sepsis could be potentially fatal. Further investigations, including bronchoalveolar lavage, revealed the presence of Pneumocystis carinii, a recognized lung pathogen in patients on long-term immunosuppressive therapy. He made a full clinical and radiological recovery following 2 weeks of co-trimoxazole therapy and was discharged home on his usual dose of maintenance immunosuppression.
Ciclosporin is a naturally occurring fungal metabolite. It has no effect on lymphocyte traffic but suppresses both humoral and cell-mediated immunity by the effect on CD4+ T cells. Ciclosporin inhibits calcium dependent signal transduction pathways downstream of the T-cell receptor, particularly the activation of several cytokine genes (Fig. 7.3). The major effect is inhibition of IL-2 production and thus CD4+ cell-dependent proliferative responses. Natural killer (NK) cell activity is also affected, because of its dependence on IL-2 production. A similar agent, Tacrolimus, is derived from a soil fungus. Although its structure is quite different from that of ciclosporin and it binds to a different intracellular protein – immunophilin, it has a similar mode of action but is 10–100 times more potent. Like ciclosporin, it inhibits IL-2, IL-3, IL-4 and interferon (IFN)-γ secretion, so preventing early activation of CD4+ T lymphocytes.
Fig. 7.3 Mechanism of immunosuppressive action of ciclosporin. In step 2 the Ciclo–Cyp complex binds to and inhibits calcineurin, a key enzyme responsible for translocation of transcription factors from the cytoplasm to the nucleus. Interruption of this event prevents gene transcription for interleukins IL-2, IL-3, IL-4, IL-5 and IFN-γ.
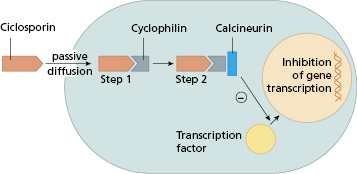
Both these agents cause a striking prolongation of graft survival and virtually all transplantation protocols now include a ‘calcineurin inhibitor’, usually in association with prednisolone and an antimetabolite such as azathioprine (see Chapter 8). Calcineurin inhibitors are partially successful both in preventing and reversing acute graft-versus-host disease following BMT. Calcineurin inhibitors have also been used in a range of autoimmune diseases mediated by helper T cells. Efficacy has been established in controlled trials in conditions such as psoriasis (Fig 11.5), uveitis and severe rheumatoid arthritis (RA). Several features are common to these reports (Box 7.1).
- These agents have a quick effect (within 2–12 weeks)
- Relapse occurs when the drug is stopped
- The long-term course of the disease maybe unaffected
- High doses are nephrotoxic and cause hypertension
The timing and dose of calcineurin inhibitors must be balanced against the risk of toxicity including nephrotoxicity, hepatotoxicity and hypertension. Other side effects include, tremor, increased susceptibility to infections, and metabolic disturbances such as hyperglycaemia. Both ciclosporin and tacrolimus are associated with an increased risk of squamous cell skin cancers and both benign and malignant lymphoproliferative diseases. This may reflect direct effects of calcineurin inhibitors or the result of altered immune surveillance of cells infected with oncogenic viruses such as Human Papilloma Virus (HPV) or Epstein–Barr virus (EBV). This risk of malignancy is not a contraindication to its use in transplantation, as the risk of such proliferation is considerably less than that of rejection of the graft.
In contrast to immunocompetent individuals who are able to contain EBV as a chronic latent infection, patients with defective cellular immunity, either secondarily to drugs or as part of a primary immunodeficiency, are unable to do so and are at risk of developing B-cell lymphoma, as in Case 7.2. EBV-induced lymphoma in transplant patients often regresses on withdrawal or reduction of immunosuppressive medication, but this has to be balanced with the attendant risk of inducing graft rejection.
 Case 7.2 Epstein–Barr virus-induced lymphoma in a transplant recipient
Case 7.2 Epstein–Barr virus-induced lymphoma in a transplant recipientA 65-year-old insulin-dependent diabetic man underwent cadaveric renal transplantation for end-stage renal failure. The immediate post-operative course was complicated by acute rejection, which was successfully reversed by steroids and then anti-thymocyte globulin. He was discharged from hospital 2 weeks later on insulin, prednisolone, azathioprine and ciclosporin (to prevent further transplant rejection), co-trimoxazole (to prevent Pneumocystis infection), erythropoietin and ranitidine. Five months later, he developed progressive dyspnoea, fever and fatigue. Clinical examination revealed bilateral lung crackles and hepatosplenomegaly. Bilateral diffuse interstitial shadowing was noted on chest X-ray. The differential diagnosis is summarized in Box 7.2. His haemoglobin was 84 g/l and he was severely leucopenic at 1.0 × 109/l. Blood cultures were sterile and a bone marrow biopsy showed normal myeloid and erythroid maturation with no acid-fast bacilli or fungi evident on special stains. A transbronchial biopsy showed no histological abnormality; PCR for acid-fast bacilli, Pneumocystis and cytomegalovirus were negative. Open lung biopsy showed fibrinous pneumonia with obstructive bronchiolitis associated with a dense cellular infiltrate of highly atypical lymphoid cells containing pleomorphic nuclei. The lymphoid cells expressed B-cell markers (CD20, CD79) and stained positively for a number of EBV gene products (EBV nuclear antigens, EBV latent membrane proteins).
The lung biopsy results were diagnostic of a B-cell lymphoma secondary to EBV. Following the diagnosis, his immunosuppressive medication was stopped but the patient died 2 weeks later from progressive respiratory failure.
- Bacterial pneumonia (unlikely at 5 months post transplant)
- Reactivation of tuberculosis
- Fungal infection (Aspergillus, Pneumocystis)
- Viral infection (cytomegalovirus)
- Epstein–Barr-virus-induced lymphoproliferative disease [Post-transplant lymphoproliferative disorder (PTLD)]
Rapamycin (sirolimus) is yet another immunosuppressive drug of fungal origin, which in combination with ciclosporin and steroids is successful in preventing renal transplant rejection. It is structurally similar to tacrolimus but has a different immunosuppressive effect: sirolimus does not inhibit calcineurin and consequently cytokine gene transcription is unimpaired. However, it inhibits T-cell proliferation induced by IL-2 and IL-4.
Despite their undoubted efficacy, immunosuppressive drug therapy is inherently unsatisfactory as illustrated in Cases 7.1 and 7.2. One method of maximizing local therapeutic immunosuppression without systemic adverse effects is the development of topical immunosuppressive agents. Tacrolimus and a related agent, pimecrolimus, are effective in ointment form in the treatment of moderate to severe eczema unresponsive to conventional therapy (see Section 4.8.2 and Case 4.11).
7.2.2 Polyclonal antibodies for prevention of responses
Antibodies can be used to prevent an immune response or to suppress ongoing immune responses.
Prevention of sensitization by removal of antigen is illustrated by the use of antibodies to the rhesus D blood group antigen. Haemolytic disease of the newborn due to rhesus incompatibility between the mother (rhesus D negative) and a rhesus D-positive fetus (see Chapter 18) is prevented by the administration of human anti-D antibodies to rhesus D-negative mothers immediately after delivery. These antibodies destroy any rhesus-positive fetal red cells, thus preventing an antibody response in the mother (Section 18.4.4).
7.2.3 Non-specific immunomodulation by intravenous immunoglobulin
Immunoglobulin replacement is essential for patients with primary antibody deficiencies (section 3.2.5) and of proven value in several forms of secondary hypogammaglobulinaemia (see Section 3.5.1). The serendipitous observation that IVIG raised the platelet count in two hypogammaglobulinaemic children with idiopathic thrombocytopenia inspired a new approach to the therapy of autoimmune disease. In these diseases, IVIG is given usually at a daily dose of 1 g/kg body weight for 1–2 days, repeated every 4–8 weeks in chronic disease. The beneficial effect of IVIG has been established by controlled trials against placebo or conventional treatment in several disorders (Table 7.3) but benefit has been claimed from open trials or anecdotal reports though these provide inconclusive evidence for general use. Some trials have shown no benefit (Table 7.3).
Table 7.3 Intravenous immunoglobulin (IVIG) as an immunomodulatory therapeutic agent
| Efficacy proven in randomized controlled trials (RCT) |
|
| Ineffective in RCT |
|
| Encouraging results in open trials/small numbers of patients |
|
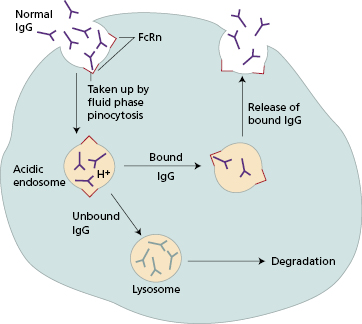
In acute immune thrombocytopenia [ITP], the rise in platelet count occurs within hours of infusion but is often only transient; in other diseases, the effect of IVIG may be long lasting. These differing patterns of response imply that different mechanisms operate. One mechanism of particular interest in autoimmune disease is the role of FcRn, the MHC class-I-related Fc receptor for IgG (also binds albumin) that protects IgG from lysosomal degradation. Blockade of FcRn by high-dose exogenous IgG is likely to result in accelerated catabolism of endogenous pathogenic IgG with consequent clinical improvement (Fig. 7.4). Fc blockade of IgG receptors on macrophages in the spleen has been shown to be one of the mechanisms involved in effectiveness in idiopathic thrombocytopenia. The mechanism of IVIg as the treatment of choice for Kawasaki’s disease in children (Case 7.3) is unknown, though neutralization of an unknown infective trigger may play a role.
 Case 7.3 Kawasaki’s disease treated with intravenous immunoglobulin
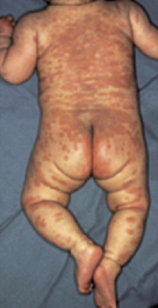
Case 7.3 Kawasaki’s disease treated with intravenous immunoglobulinA 2-year-old boy was admitted to hospital with a 7-day history of high fever, lymphadenopathy, conjunctivitis and an erythematous exfoliative rash affecting his trunk and extremities (see Fig 7.5). On the basis of the characteristic clinical picture, a clinical diagnosis of Kawasaki’s disease (also known as acute mucocutaneous lymph node syndrome), an acute vasculitic disorder of infants affecting small and medium-sized blood vessels, was made. Other infective causes of a similar clinical presentation were excluded on the basis of negative blood and urine cultures. The results of initial investigations were as follows:
- Hb 110 g/l (NR 120–150)
- White cell count 14 × 109 (NR 4–11)
- Platelets 550 × 109 (NR 250–400)
- C-reactive protein 80 mg/l (NR < 10)
Fig. 7.5 Infant with Kawasaki’s disease with an erythematous, predominantly truncal rash. With permission from Alexander F.Freeman and Stanford T. Shulman.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree