Fig. 20.1
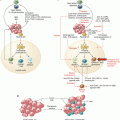
The ligands for PD-1 are PD-L1 and PD-L2. PD-L1 and PD-L2 may also bind to B7.1 and RGMb
20.3 Initial Studies with Single Agent Immune Checkpoint Inhibitors
In the first-in-human phase I clinical trial with nivolumab, a fully human immunoglobulin G4 (IgG4) against human PD-1 previously known as MDX-1106 or BMS-936558, 39 patients with advanced metastatic NSCLC, melanoma, renal cell carcinoma, colorectal carcinoma, or castrate-resistant prostate cancer were treated in four escalating single dose cohorts of 0.3, 1, 3, and 10 mg/kg [23]. All patients had progressive and refractory disease, with a median of four of prior therapies. The primary objectives were safety and tolerability of single dose therapy. Patients without tumor progression or adverse events grade 3 or higher received additional doses at weeks 12 and 16, with the option of repeated treatment at 3 months. The treatment was well tolerated, with no dose-limiting toxicities after the single dose. Twelve patients received multiple doses of nivolumab after achieving stable disease or tumor regression after the initial dose. This study was followed by a dose-expansion cohort trial in patients with the same malignancies using 1, 3, or 10 mg/Kg every 2 weeks for up to 2 years in the absence of unacceptable adverse events, complete response (CR), or progressive disease (PD) [24]. The updated report of the NSCLC cohort included 129 patients, of which 70 (54.3%) had been previously treated with three or more prior lines of systemic regimens [25]. The treatment was well tolerated, with grade 3 or 4 treatment-related adverse events (TAEs) occurring in 14% of patients. Nevertheless, there were three treatment-related deaths due to pneumonitis. The ORR was 17% across all doses, without differences between squamous and non-squamous histologies. The median PFS was 2.3 months, with 1-year and 2-year PFS of 22% and 9%, respectively. The median, 1-year, 2-year, and 3-year OS were 9.9 months, 42%, 24%, and 18%, respectively. The 0.3 mg/kg cohort was associated with the best outcomes, with ORR of 24.3%, median OS of 14.9 months, and 1-year, 2-year, and 3-year OS rates of 56%, 42%, and 27% respectively.
The Keynote-001 study assigned 495 NSCLC patients to different doses of pembrolizumab, previously known as MK-3475, a humanized IgG4 monoclonal antibody against PD-1 [26]. The treatment was well tolerated, with grade 3 or higher TAEs occurring in 47 patients (9.5%). The ORR was 19.4%, with median PFS and OS of 3.7 months and 12 months, respectively. The percentage of neoplastic cells showing membranous staining for PD-L1 by immunohistochemistry was used as a biomarker selection in the 182 patients assigned to the training group. PD-L1 expression in at least 50% of tumor cells was selected as the cutoff. The ORR, median PFS, and OS for patients PD-L1 expression of at least 50% in the validation cohort were 45.2%, 6.3 months, and not reached, respectively. There were no differences in efficacy among the regimens tested. Among the 824 patients who had samples evaluated for the study, the prevalence of PD-L1 scores of 50% or more, 1–49%, and less than 1% were 23.2%, 37.6%, and 39.1%, respectively.
In the initial study with atezolizumab, a human IgG1 monoclonal antibody against PD-L1 previously known as MPDL-3280A, 277 patients with solid tumors were treated with escalating doses by intravenous infusion every 3 weeks for up to 16 cycles of 1 year [27]. Among the 53 patients with NSCLC, the ORR and 24-week PFS were 23% and 45%, respectively. There was a correlation between responses and PD-L1 expression in the pretreatment specimens, which for NSCLC reached statistical significance in the tumor-infiltrating immune cells (ICs) but not in tumor cells (TCs). Most patients with tumor progression had no PD-L1 expression in TCs or ICs, and the growing tumors had a pattern or immune ignorance with little to no tumor-infiltrating immune cells, nonfunctional immune response with immune infiltrate expressing minimal to no expression of PD-L1, or excluded immune infiltrate which was located around the outer edge of the tumor.
20.4 Randomized Clinical Trials in Previously Treated Patients
The encouraging results from single agent monoclonal antibodies against PD-1 or PD-L1 in patients with advanced stage NSCLC led to randomized clinical trials using docetaxel 75 mg/m2 every 3 weeks as the control arm. All studies had OS as the primary endpoint.
In the CheckMate-017 trial, 272 patients with advanced squamous cell lung cancer with one prior line of therapy were randomized to nivolumab 3 mg/kg every 2 weeks or docetaxel [28]. Nivolumab was better tolerated than docetaxel, with no new safety concerns identified. Treatment with nivolumab was associated with increased ORR (20% vs 8%, P = 0.008), median PFS (3.5 vs 2.8 months, hazard ratio [HR] 0.62, P < 0.001), and median OS (9.2 vs 6.0 months, HR 0.59, P < 0.001) compared to docetaxel. The benefit from nivolumab on both PFS and OS was independent of PD-L1 expression in TCs. The CheckMate-057 had a similar design and randomized 582 patients with advanced NSCLC to standard dose of either nivolumab or docetaxel [29]. Patients were required to have one prior line of platinum-based chemotherapy doublet, and those with known EGFR mutation or ALK translocation were allowed to have received one additional line of tyrosine kinase inhibitor. Similarly to the CheckMate-017 trial, nivolumab was better tolerated than docetaxel and associated with improved ORR (19% vs 12%, P = 0.02). The median PFS, however, was numerically superior for the docetaxel arm (2.3 vs 4.2 months; HR 0.92, P = 0.39), whereas the 1-year PFS favored nivolumab (19% vs 8%). Both the median (12.2 vs 9.4 months; HR 0.73, P = 0.002) and 1-year OS (51% vs 39%) were better with nivolumab. The results of these two studies led to the approved nivolumab in previously treated patients with squamous cell and non-squamous cell histologies.
The Keynote-010 randomized 1034 patients with previously treated NSCLC- and PD-L1-positive tumors to docetaxel, pembrolizumab 2 mg/kg, or pembrolizumab 10 mg/kg every 3 weeks [30]. Pembrolizumab was better tolerated than docetaxel. There were no significant differences in OS between the two pembrolizumab arms, and the dose of 2 mg/kg was chosen for further studies. When compared to docetaxel, pembrolizumab 2 mg/kg was associated with increased ORR (18% vs 9%, P = 0.0005), similar median PFS (3.9 vs 4 months, HR 0.88, P = 0.07), and improved median OS (12.7 vs 8.5 months, HR 0.71, P = 0.0008). The benefit from pembrolizumab at the recommended dose was more pronounced in patients with PD-L1 proportion score of at least 50%, with increased ORR (30% vs 8%, P < 0.0001), median PFS (5 vs 4.1 months, HR 0.59, P = 0.0001), and median OS (14.9 vs 8.2 months, HR 0.59, P = 0.0002). On subgroup analysis, the OS favored pembrolizumab in all variables except for EGFR-mutant patients (HR 0.88, 95% confidence interval [CI] 0.45–1.70), where a trend toward worse PFS was also found (HR 1.79, 95% CI 0.94–3.42).
The POPLAR was a randomized phase 2 trial where 287 patients with advanced stage NSCLC previously treated with one line of platinum-based chemotherapy were randomized to atezolizumab 1200 mg or docetaxel at standard dose every 3 weeks [31]. PD-L1 was scored for both TCs and ICs. Atezolizumab was well tolerated, with no new safety signals. Although atezolizumab was not associated with differences in ORR (15% vs 15%) or median PFS (2.7 vs 3 months, HR 0.94; 95% CI 0.72–1.23), the primary endpoint was met with a significant improvement in OS in the experimental arm (12.6 vs 9.7 months, HR 0.73, P = 0.04). The OS benefit for atezolizumab was observed only in patients with at least TC1 or IC1. The OAK was a phase 3 study which randomized 1125 patients with advanced NSCLC previously treated with one or two lines of chemotherapy to atezolizumab 1200 mg or docetaxel [32]. Atezolizumab was better tolerated than docetaxel with fewer grade 3 or 4 adverse events (34% vs 54%). The study showed no improvement from atezolizumab in ORR or median PFS (2.8 vs 4 months, HR 0.95; 95% CI 0.82–0.10) in the intent to treat population. Nevertheless, the median OS was significantly longer with atezolizumab (13.8 vs 9.6 months, HR 0.73. P = 0.0003). The improved OS was observed regardless of PD-L1 expression levels, including patients with TC0 and IC0 (12.6 vs 8.9 months, HR 0.75, P = 0.02), TC1-3 or IC1-3 (15.7 vs 10.3 months, HR 0.74, P = 0.01), and TC3 or IC3 (20.5 vs 8.9 months, HR 0.41, P < 0.0001). The median OS favored atezolizumab in all prespecified groups except for patients with EGFR mutation (HR 1.24; 95% CI 0.71–2.18).
20.5 First-Line Therapy
With the established safety and efficacy of single agent immune checkpoint inhibitors in previously treated patients, with some patient achieving an unprecedented prolonged survival, the next step was to evaluate this strategy in the first-line setting.
The CheckMate-012 is a phase I multi-cohort study evaluating the use of nivolumab alone or in combination with ipilimumab, platinum-based chemotherapy, erlotinib, or bevacizumab maintenance in patients with advanced NSCLC. In the first-line nivolumab monotherapy arm, ORR was observed in 12 out of 52 patients (23%), with 14 additional patients achieving SD for a confirmed disease control rate (DCR) of 50% [33]. The median PFS and OS were 3.6 months and 19.4 months, respectively. Although patients with PD-L1 less than 1% had lower responses compared to those with PD-L1 positive (14% vs 50%), there were no significant differences in 1-year OS (79% vs 83%). In the combined immune checkpoint study, 78 chemotherapy naïve patients with advanced NSCLC were randomized to nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 or 12 weeks [34]. Both arms had a tolerable safety profile. The ipilimumab every 12-week cohort was associated with numerically superior confirmed ORR (47% vs 38%) and median PFS (8.1 vs 3.9 months). Nevertheless, the ipilimumab every 6 weeks regimen was chosen for further development in NSCLC based on observation from studies in melanoma and small cell lung cancer where greater exposure to ipilimumab was associated with improved activity of the combination. Pooled results from both cohorts showed that there were 12 confirmed and 1 unconfirmed response among the 13 patients with PD-L1 ≥50%. In the third cohort of CheckMate-012 reported, 56 patients with previously untreated advanced NSCLC were treated with nivolumab 10 mg/kg plus a platinum-based doublet chemotherapy every 3 weeks for four cycles followed by maintenance nivolumab monotherapy until tumor progression or unacceptable toxicity [35]. The chemotherapy regimens used were gemcitabine plus cisplatin for squamous carcinoma, pemetrexed plus cisplatin for non-squamous carcinomas, and carboplatin plus paclitaxel for any histology. An additional arm included was nivolumab 5 mg/kg plus carboplatin and paclitaxel. There were 35 patients (95%) with TAE of any grade and 25 (45%) with grade 3 or 4 toxicity. Grade 3 or 4 pneumonitis was observed in four patients (7%). The confirmed ORR, median PFS, median OS, 1-year OS, and 2-year OS were 33–47%, 4.8–7.1 months, 11.6 to not reached, 50–87%, and 25–62%, respectively. The outcomes were considered particularly promising for the 14 patients receiving nivolumab 5 mg/kg plus carboplatin and paclitaxel, with confirmed ORR of 43%, estimated duration of response of 19.6 months, median OS not reached, 1-year OS of 86%, and 2-year OS of 62%.
In the randomized open-label phase 2 Keynote-021 trial, 123 patients with chemotherapy naïve advanced stage non-squamous NSCLC were randomized to carboplatin plus pemetrexed with or without pembrolizumab [36]. The pembrolizumab arm was associated with increased grade 3 or higher adverse effects (40% vs 26%). The primary endpoint of objective response was met with a significant increase in the ORR for the triplet therapy (55% vs 29%, P = 0.001). Although the median PFS was numerically higher for the pembrolizumab arm (13.0 vs 8.9 months), there was no difference in OS, with median not reached and estimated 6-month OS of more than 90% in both groups.
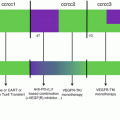
The Keynote-024 was an open-label randomized phase 3 trial comparing pembrolizumab 200 mg every 3 weeks to platinum-based chemotherapy in previously untreated patients with advanced stage NSCLC and PD-L1 expression of ≥50% in tumor cells (Fig. 20.2) [37]. Patients with EGFR mutation or ALK translocations were excluded from the study. The chemotherapy options were cisplatin plus pemetrexed, carboplatin plus pemetrexed, cisplatin plus gemcitabine, carboplatin plus gemcitabine, and carboplatin plus paclitaxel. Among the 1934 patients screened, 1653 had samples evaluated for PD-L1, of which 500 (30.2%) had PD-L1 proportion ≥50%. The primary endpoint was PFS. There were 305 patients eligible for the study, with 154 randomized to pembrolizumab and 151 to chemotherapy. The most common chemotherapy used was carboplatin plus pemetrexed. Pembrolizumab was associated with decreased incidence of adverse events of any grade (73.4% vs 90%) and grade 3 or higher (26.6% vs 53.3%). There were 45 (29.2%) immune-mediated adverse events in patients treated with pembrolizumab, most commonly hypothyroidism (9.2%), hyperthyroidism (7.8%), and pneumonitis (5.8%). Adverse events leading to death were found in one patient (0.6%) treated with pembrolizumab and three patients (2%) treated with chemotherapy. ORR was higher in patients treated with pembrolizumab (44.8% vs 27.8%). The median FPS was significantly longer in the pembrolizumab group (10.3 vs 6 months, HR 0.50, P < 0.01). Although the median OS was not reached in either group, the estimated percentage of patients alive at 6 months was also higher in patients treated with pembrolizumab (80.2% vs 72.4%). The results from the Keynote-024 established a new standard of care for patients with previously untreated advanced stage NSCLC (wild type for EGFR-activating mutations or ALK translocations) and tumor PD-L1 ≥50%, with better toxicity profile and improved ORR and PFS compared to standard chemotherapy (Fig. 20.3).
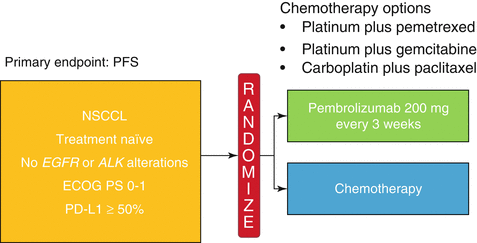
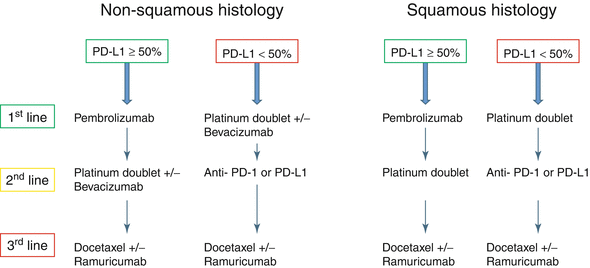

Fig. 20.2
Design for the Keynote-024. Patients with untreated stage IV NSCLC were randomized to pembrolizumab or chemotherapy. The chemotherapy choices included cisplatin plus pemetrexed, carboplatin plus pemetrexed, cisplatin plus gemcitabine, carboplatin plus gemcitabine, and carboplatin plus paclitaxel

Fig. 20.3
New algorithm for the treatment of advanced stage NSCLC without oncogene-driven tumors, according to PD-L1 expression
20.6 Predictors for Response to Immune Checkpoint Inhibitors
Since prolonged tumor control from immune checkpoint inhibitors is achieved in only a small percentage of patients, there has been a great interest in defining the predictors for benefit.
The most studied biomarker is PD-L1, which is detected by immunohistochemistry (IHC) and is commonly upregulated on the surface of tumor cells. Multiple studies have shown an association between tumor response and PD-L1 expression, although patients with no PD-L1 expression also achieve benefit. In a meta-analysis including 1567 NSCLC patients treated with immune checkpoint inhibitors, the ORR was 29% for 652 PD-L1-positive tumors and 13% for PD-L1-negative tumors (relative ratio [RR] 2.08, 95% CI 1.49–2.91, P < 0.01) [38]. The differences in ORR were also observed for both squamous (29% vs 14%, RR 2.12; 95% CI 1.37–3.29, P < 0.01) and non-squamous histologies (32% vs 11%, RR 3.14; 95% CI 2.15–4.54, P < 0.01). Nevertheless, although the 24-week PFS was significantly higher in patients with PD-L1-positive tumors (35% vs 26%, P < 0.01), there were no differences in 1-year OS (28% vs 27%, P = 0.39). Similar findings were observed in an analysis of 511 NSCLC patients treated on seven studies, where the ORR was higher in patients with PD-L1-positive tumors (23.2% vs 14.5%) [39].
Direct assessment of PD-L1 expression on tumor cells represents the most logic and straightforward biomarker for prediction of benefit from immune checkpoint inhibitors against PD-1 or PD-L1. However, there are several factors that should be taken into account for the proper interpretation of PD-L1 results, including the mechanisms of PD-L1 upregulation, presence of tumor-infiltrating cells (TILs), and methodology of IHC testing.
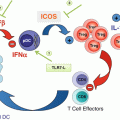
PD-L1 expression in tumor cells may be constitutive or adaptive (inducible). These mechanisms are not mutually exclusive and may coexist in the same tumor microenvironment (TME) [40]. Constitutive PD-L1 expression occurs through alterations in oncogenic pathways including amplification of 9q24 which contains the locus for PD-L1, PD-L2, and JAK-2 (PDJ amplicon), PTEN deletions, PI3K or AKT mutations, and MYC overexpression [41]. PD-L1 expression may also be induced by interferons (INFs), particularly INFγ, which is produced by activated Th1 helper CD4 cells, activated CD8 cells, and natural killer (NK) cells. INFγ results in PD-L1 expression in surrounding cells with interferon receptors including cancer cells and cells within the TME such as myeloid-lineage, stromal, and T cells (Fig. 20.4). Inducible expression occurs more commonly than constitutive and is characterized by patchy patterns of PD-L1 at the interface between tumor cells and TILs [42]. In contrast, constitutive PD-L1 expression is characterized by a more homogeneous PD-L1 staining in most cells.
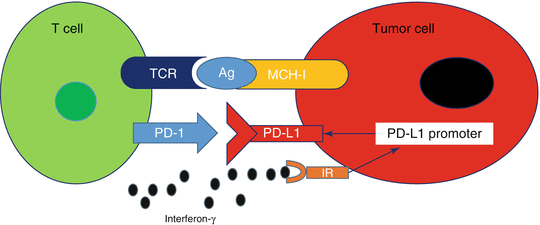

Fig. 20.4
Adaptive immune resistance. Binding of interferon-γ to the interferon receptor (IR) leads to increased PD-L1 expression
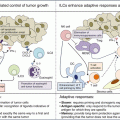
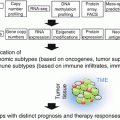
Tumors may be classified into four distinct groups based on the PD-L1 status and presence of TILs (Fig. 20.5) [43]. Type I tumors have TILs and are PD-L1 positive, whereas type III tumors have PD-L1 expression in the absence of TILs. Types II and IV have PD-L1 negative with TILs absent in the former and present in the latter. Type I tumors represent the adaptive mechanism from the tumor cells expressing PD-L1 in response to INFγ secretion by the infiltrating immune cells, whereas type III tumors represent the constitutive PD-L1 expression, which is not related to the interaction with INFγ secreting cells. Type II tumors with neither TILs nor PD-L1 expression may represent immune ignorance, whereas in type IV tumors, the presence of TILs without induction PD-L1 in the tumor cells may indicate the presence of immune tolerance [44].
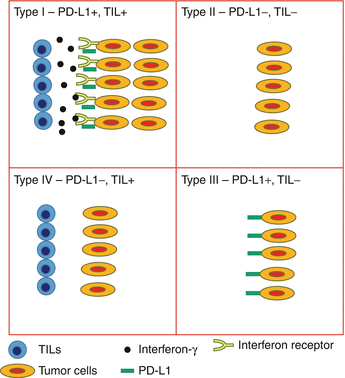

Fig. 20.5




Tumor classification based on PD-L1 expression and TILs. Type I is characterized by the presence of PD-L1-positive tumor cells at the interface with TILs, representing the adaptive expression in response to cytokines secreted by the infiltrating immune cells. This type of tumor is the most likely to respond to immune checkpoint blockade. Type III represents the constitutive PD-L1 expression by the tumor cells in the absence of TILs. It is unclear whether constitutive PD-L1 expression predicts for benefit from monoclonal antibodies against PD-1 or PD-L1. Types II and IV are characterized by PD-L1-negative tumors, with the former most likely caused by immune ignorance whereas the latter may be due to other immunosuppressive mechanisms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree