and Baorui Liu1
(1)
The Comprehensive Cancer Centre of Drum Tower Hospital, Medical School of Nanjing University and Clinical Cancer Institute of Nanjing University, Nanjing, 210008, China
9.1 Introduction
The term “checkpoints” refers to a broad spectrum of either co-receptors or ligands that are widely expressed by immune cells. Importantly, such checkpoints regulate immune cells’ activation. The “inhibitory checkpoints” represent those molecules that play an important role in preventing over-activation of the immune system and are important to maintaining self-tolerance. In this way, immune attack by the host immune system can be prevented. Conversely and in the context of the tumor-immune environment, co-inhibitory receptors may pose a threat to the host’s health by preventing an immune response against these malignancies. Both co-inhibitory receptors and ligands are highly expressed in a large number of malignancies. This high expression allows for successful evasion of antitumor immune responses. One of the most promising tumor immunotherapy strategies is to interrupt these immune “brakes” by blocking antibodies that prevent interactions between receptors and their cognate ligands. As shown by recent clinical trials targeting either the PD-1/PD-L1 or CTLA-4, pathways has yielded exciting results. However, there remains very limited study and understanding on gastric cancer when compared to other malignancies such as melanoma or lung cancer.
As such, this chapter seeks to first summarize clinical experiences and outcomes that are based on the use of anti-PD-1, anti-PD-L1, and anti-CTLA-4 monoclonal antibodies. We will then discuss how a selection of promising inhibitory checkpoint function, with a particular focus on their expression landscape in gastric cancer. Finally, we will highlight related clinical studies as well as preclinical research, concentrating on strategies that have been used to disturb them in order to enhance their immunotherapeutic efficacy against gastric cancer.
9.2 PD-1/PD-L1 Axis
9.2.1 Introduction to PD-1/PD-L1
PD-1 is also known as CD279 and is an immunoglobulin superfamily surface molecule sharing homology with both CD28 and CTLA-4 [1, 2]. PD-1 is expressed on activated T cells, B cells, NK cells, and myeloid-derived cells [3–5]. Its expression is relatively low on naïve T cells but can be induced by TCR signaling as well as by cytokines such as IL-2, IL-7, IL-15, and IL-21 [6]. PD-L1 is a ligand for PD-1; it is also known as B7H1 or CD274 and shares significant homology with B7-1 (CD80). It also has a broad distribution on both hematopoietic cells such as T cells, B cells, dendritic cells (DCs), myeloid cells, as well as non-hematopoietic cells such as tumor cells [7]. It is constitutively expressed at low levels, and it is robustly induced by Th1 cytokines such as IFN-γ and TNF-α [8, 9]. PD-L2 is another ligand for PD-1, sharing homology with B7-2 (CD86). It is expressed on DCs and competes with PD-L1 to inactivate T cell functioning.
When compared with PD-L1, much less is known about PD-L2, beyond the fact that it is expressed at much lower levels [10]. PD-1 itself has been shown to recruit the tyrosine phosphatases SHP-2 and SHP-1 to the membrane where PD-1 ligation takes place. As a result, signaling through CD3-TCR and CD28 are inactivated, leading to T cell exhaustion and apoptosis [11]. Simultaneously with PD-1 ligation, PD-L1-positive tumors receive an anti-apoptotic signal, preventing them from succumbing to lysis by cytotoxic T lymphocytes (CTLs) [12] (Fig. 9.1). The past 5 years has seen successful use of a PD-1 blocking antibody in clinical trials and has raised the plausibility of immunotherapy in various kinds cancers [13–18]. Clinical data have revealed that up to 40% of patients with advanced-stage melanoma experienced measurable responses upon treatment with pembrolizumab or nivolumab, two PD-1 blocking antibodies approved by the FDA in 2014. In 2015, nivolumab was also approved for the treatment of squamous cell lung cancer, which is resistant to chemotherapy. Nivolumab and pembrolizumab have since been expanded to the treatment of additional cancers, including NSCLC, RCC, bladder, ovarian, and other malignancies [19]. Promising results have also been shown in patients with hematologic malignancies such as Hodgkin lymphoma [18].
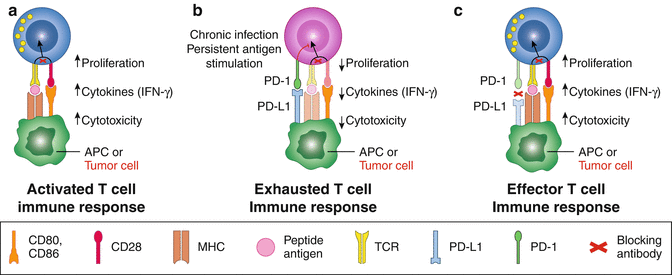

Fig. 9.1
PD-1/PD-L1 in cancer therapy Cancer Med, (2013) 2(5): 662–673. (a) Activation of T cells by CD28-CD80/86 signal. (b) Tolerence of T cells by PD-1-PD-L1 signal. (c) Reactivation of T cells by PD-1-PD-L1 blocking (Adapted from PD-1/PD-L1 in cancer therapy Cancer Med, (2013) 2(5): 662–673)
9.2.2 PD-1/PD-L1 in Gastric Cancers
When turning the attention to examining the evidence for PD-1 and PD-L1 blockade in gastric cancers, in this chapter, the clinical significance as well as clinical studies of this pathway in the treatment of gastric cancers will be summarized. Besides, some of the future strategies targeting this pathway will be discussed.
9.2.2.1 Preclinical Studies
Preclinical studies investigating PD-1 and PD-L1 blockade therapy for gastric cancer have focused predominantly on mouse models and have yielded limited results. Some engrafted human tumor cells into T cell-deficient, nude mice to explore their efficacy. However, this model has limited the use for immunotherapy, since the effect of antibody blockade depends entirely on the interaction between immune and tumor cells. Given this, other studies have focused on immune-competent mice that have been modified to express human immune system components [20, 21]. Other strategies also use human PBMC to reconstitute the immune system of mice in order to validate the efficacy of the immune blocking antibody. Despite this wide array of approaches, models featuring human tumors inoculated onto mice have different signatures from those found in humans. It has also been suggested that most mouse models constitutively express PD-L1 [22]. With these limitations in mind, the following discussion will focus predominantly on human studies.
9.2.2.2 Clinical Significance of PD-1/PD-L1 in Gastric Cancers
Expression, Clinical Associations, and Prognosis
A variety of studies have described the clinical significance of the PD-1/PD-L1 pathway in gastric cancer. In these studies, the expression rates of PD-L1 or PD-1 in gastric cancer tumor tissues or peripheral blood were measured along with their correlated clinical-pathological features. These studies also then described the prognostic value of these biomarkers. Examples of these studies are summarized below and can be found in Tables 9.1 and 9.2.
Table 9.1
Expression of PD-L1 in gastric cancer patients
Time | Author | Country | Journal | Sample size | Positive rate | Associated characteristics | Prognostic factor |
|---|---|---|---|---|---|---|---|
2016 | Changping Wu | China | Acta histochemica | 102 | 42.2% | Tumor size; invasion; lymph node metastasis | Poor survival |
2016 | Cristin Boger | Germany | Oncotarget | 465 | 30.1% on tumor cells 88.4% on immune cells | MSI; EBV; PIK3CA mutation Men | Better survival |
2016 | Elizabeth D Thompson | USA | Gut | 34 | 12% on tumor cells 44% immune stroma | CD8+ T cell density | Poor survival |
2015 | Kei Muro | Multicenters | ASCO GI symposium | 162 | 40% | – | – |
2015 | Lin Zhang | China | Int J Clin Exp Pathol | 132 | 50.8% | Tumor size >5 cm | Poor survival |
2014 | Kim J.W | Korean | Gastric Cancer | 243 | 43.6% | Less advanced stage; intestinal type; well differentiated | Better survival |
2014 | Jing ying Hou | China | Experimental and Molecular Pathology | 111 | 63% 71% | Lymph node metastase | Poor survival |
2014 | Zhixue Zheng | China | Chin J Cancer Res | 80 | Low in 41.2%; high in 58.8% (serum) | Differentiation lymph node metastasis | Better survival |
Table 9.2
PD-1 expression on immune cells of gastric cancer patients
Time | Author | Country | Journal | Sample size | Positive rate | Associated characteristics | Prognostic factor |
|---|---|---|---|---|---|---|---|
2016 | Japanese | Gastric Cancer | 105 | – | PD-L1 expression; Foxp 3 expression | Poor prognosis | |
2016 | Japanese | Surg Today | – | – | TIM-3 expression; reduced IFN-ϒ production | – | |
2016 | Cristin Boger | Germany | Oncotarget | 465 | 53.8% | PD-L1 expression | – |
2015 | Seigo Takaya | Japanese | Yonago Acta medica | 33 | 17.7% on CD4 15.5% on CD8 | Post surgery; LAG-3 expression; | – |
2013 | Hiroaki Saito | Japanese | Journal of Surgical Oncology | 40 | 31~73.4% | Tumor progression; reduced IFN-ϒ production | – |
Wu et al. examined PD-L1 protein expression in 102 cases of gastric cancer using immunohistochemical staining. They found that PD-L1 is detectable in 42.2% of gastric carcinoma tissues and not in healthy tissues. Furthermore, PD-L1 expression is significantly correlated with tumor size, invasion, and lymph node metastasis. Taken together, these results show that PD-L1 could be used as an independent factor to evaluate the possible outcomes of gastric cancer [23].
Hou et al. also used immunohistochemistry to evaluate FOXP3 and PD-L1 expression in tumor sections obtained from 111 gastric cancer patients. Co-expressions of PD-L1 and FOXP3 were found in these tissue samples. Moreover, the expressions of PD-L1 and FOXP3 were found to be correlated with lymph node metastasis and clinical-pathological stages, indicating poor prognosis. This study provided evidence for the idea that depleting Tregs in combination with PD-L1 blockade might improve immunotherapeutic efficacy in gastric cancer [24].
In another study, Kim et al. examined post-tumor resections obtained from 243 gastric cancer patients. PD-L1 was observed in 43.6% of the patients and was related to intestinal type, well-to-moderate differentiation and a less advanced stage. PD-L1 expression was found to be related to better disease-free survival (DFS) (5-year DFS rate: 82.6% vs. 66.9%) and overall survival (OS) (5-year OS rate: 83.0% vs. 69.1%) in gastric cancer. More importantly, patients within higher infiltration of CD3+ cells showed better survival outcomes. In a subsequent multivariate analysis, PD-L1 expression had a better prognostic impact on survival, independent of other clinical variables [25].
Additionally, Zhang et al. reported that PD-L1 expression was observed in 50.8% of 132 surgically resected gastric cancer specimens. There was no relationship between the expression of PD-L1 and any clinical-pathological variables. However, patients with larger tumor size (>5 cm) had a higher positive rate of PD-L1 expression. Also, PD-L1 positively expressing patients had poorer long-term survival rates (5-year survival rates: 83.1% vs. 50.7%) [26].
Elizabeth et al. focused on 34 resections obtained from primary invasive gastroesophageal junction (G/GEJ) adenocarcinomas and performed immunohistochemistry for PD-L1 and CD8 expression. Of these, 12% showed membrane expression of PD-L1 on tumor cells, while 44% showed PD-L1 expression within the immune stroma itself. Increasing CD8+ infiltrations were associated with an increasing percentage of tumor and stromal PD-L1 expression, both within tumors and immune stroma. This finding indicated an adaptive immune resistance pattern. Moreover, they found both tumor, and immune stromal PD-L1 expression was associated with a worsening of disease-free and overall survival rates [27].
Christine et al. also used an immunohistochemical approach to investigate the expression of PD-L1 and PD-1 in samples taken from 465 gastric cancer patients. PD-L1 expression was found in the tumor cells of 140 gastric cancer patients (30.1%) and in 9 (60%) liver metastases from these same patients. PD-L1 expression was also found in the immune cells from 411 (88.4%) of gastric cancer patients and 11 (73.3%) liver metastases from these same patients. PD-L1 expression was significantly more abundant in the following: samples obtained from men, Her2/neu positive, Epstein-Barr virus positive, microsatellite unstable, PIK3CA-mutated, and the proximal, unclassified, papillary gastric cancer. High PD-L1 expression was independently associated with better clinical outcome [28].
In addition to PD-L1 membrane staining, Zheng et al. also examined PD-L1 expression in the circulation of gastric patients using an enzyme-linked immunosorbent assay (ELISA). This approach was tested on 80 advanced gastric cancer patients and 40 health controls. Researchers found that circulating PD-L1 was upregulated in gastric cancer patients and correlated with lymph node metastasis and tumor differentiation. In this study, patients with high PD-L1 expression had a better prognosis than patients with low PD-L1 expression [29].
There are also emerging work examining the relationship between genetic polymorphism of PD-1/PD-L1 and gastric cancer. For instance, Savabkar et al. evaluated the association of PD-1.5 C/T polymorphism in 122 gastric cancer patients and 166 healthy controls. They found that the frequencies of PD-1.5CT genotypes were higher in gastric cancer patients [30]. Other work has also indicated that some PD-L1 genotypes have a strong correlation with the clinical-pathological features of gastric cancer [31].
Despite the relatively stable expression of PD-L1 in the tumor tissue of gastric cancer, there is no definitive conclusion as to whether PD-L1 expression can predict a good or poor disease prognosis. It is likely to be much more complicated in the post-curative resection setting or other specific situations such as infection with the Epstein-Barr-virus status, microsatellite instability, and/or high mutation load gastric cancers. Future work will require expanded cases and additional research to better understand PD-L1’s role in the development and prognosis of gastric cancer.
There are fewer studies examining PD-1 expression relative to PD-L1 in gastric cancer patients. For instance, Hiroaki et al. found that PD-1 expressions on both CD4+ T cells and CD8+ T cells were significantly higher in gastric cancer patients than in normal controls. In addition, PD-1 expression on CD4+ T cells and CD8+ T cells in advanced-stage patients was significantly higher than in early-stage patients. Finally, they also found that less IFN-γ was produced by PD-1 positive T cells than by PD-1-negative T cells, indicating that PD-1 upregulation may play a role in the immune evasion capacity of gastric cancer [32].
In another study, Seigo et al. evaluated PD-1 expression on both CD4+ and CD8+ T cells obtained pre- and postoperatively from gastric cancer patients. Total lymphocyte count decreased rapidly postoperation. In contrast, PD-1+CD4+ and PD-1+CD8+ T cells showed a significant increase postoperation and returned to preoperative levels on day 30. Collectively, these results showed that PD-1 expression was upregulated on T cells after surgery. Moreover, it could be related to the impaired cell-mediated immunity seen in gastric cancer [33].
Christine et al. demonstrated that PD-1 was expressed in tumor-infiltrating lymphocytes in 53.8% of gastric cancer patients and in 73.3% of those with liver metastases. PD-1-positive immune cells were frequently observed in either intra-tumor lymphocyte aggregates or lymph follicles. Moreover, PD-1 expression in TILs was significantly correlated with PD-L1 expression in tumor microenvironments (TME). Higher PD-L1/PD-1 expression was also associated with better clinical outcome. Furthermore, there was a correlation between PD-L1/PD-1 expression and distinct clinical-pathological characteristics such as EBV status, MSI, Her2/neu positivity, and PIK3CA mutation status. These characteristics may instruct the use of immune checkpoint treatment strategies in the treatment of PD-L1-positive gastric cancer [28].
Takano et al. found PD-1 expression on CD8+ T cells obtained from gastric cancer patients was significantly correlated with Tim-3 expression in peripheral blood samples. T cells positive for both PD-1 and Tim-3 had significantly reduced production of IFN-γ than negative cells. This result indicates that PD-1, together with other immune inhibitory checkpoints, mediates the immune tolerance of gastric cancer [34].
Eto et al. evaluated PD-1 expression on tumor tissue obtained from 105, post-curative surgery, gastric cancer patients. PD-1 was found to be correlated with both PD-L1 and Foxp3 expression. Moreover, PD-1-positive patients had poorer survival rates when compared with PD-1-negative patients (3-year DFS: 36.1% vs. 64.7%). In this way, PD-1 expression can be used as an independent prognostic indicator of gastric cancer [35].
Molecular Classification of Gastric Cancer and PD-1/PD-L1 Blockade Strategy
The Cancer Genome Atlas (TCGA) project has classified gastric cancer into four molecular subtypes according to the following characteristics: (1) EBV, tumors positive for Epstein-Barr virus; (2) MSI, microsatellite unstable tumors; (3) GS, genomically stable tumors; and (4) CIN, tumors with chromosomal instability [36]. Christine et al. found that PD-L1 expression in tumor cells was both more intense and extensive in EBV-positive, MSI, papillary-type, and unclassified gastric cancers. In particular, MSI-GCs have peculiar histological MSI features and often showed high PD-L1 expression in tumor cells. Moreover, four different PD-L1 expression patterns were observed: (1) EBV-positive GCs with a heterogeneous, “patchy” expression pattern with a striking accumulation of PD-L1-positive tumor cells around larger blood vessels; (2) MSI-GC were mainly PD-L1 positive at the interface between neoplastic and nonneoplastic tissues; (3) papillary-type GCs often showed PD-L1 positivity within the fibrovascular connective tissue cores and the intra-tumor necrosis; and (4) other cases showed no distinct PD-L1 distribution pattern and were classified as “patternless” [28].
EBV-GCs
Globally, there are approximately 80,000 new cases each year of EBV-associated gastric cancer (EBVaGC), comprising almost 10% of total gastric cancer cases worldwide [37]. Recent evidence has suggested that the immune system may play an important role in the development of EBVaGC, since EBV infection is always accompanied by a high degree of immune cell infiltration [38]. Studies have also revealed that most genetic changes in EBVaGC occur in immune response genes, including significant amplification of PD-L1 and PD-L2 [38]. Immune responses have also been shown to be negatively regulated via PD-1 ligation [39]. Prior investigations have shown PD-L1 overexpression on the cell surface of lymphoma, which inhibited lysis of infected, malignant cells by cytotoxic T cells [40]. Therefore, it is likely that EBV-reactive immune cells may be dysfunctional due to the acquired immune resistance mediated by the PD-1/PD-L1 pathway.
Given this, it was hypothesized that inhibition of the PD-1/PD-L1 pathway might augment antitumor immune responses. In a recent study from our lab, the PD-L1 expression on CD3+ T cells that had infiltrated gastric tumor tissues was evaluated. The results indicated that PD-L1 was more prevalent on cells obtained from EBVaGC patients than from patients with EBV nonassociated gastric cancer (EBVnGC) (64% vs. 15%, respectively). Moreover, PD-1 was found to be upregulated on EBV-LMP2A-specific CD8+ T cells. Finally, using CRISPR-Cas9 interference with the PD-1 gene of cytotoxic T cells resulted in a significant enhancement of IFN-γ production and killing ability of T cells.
MSI-GCs
Recently, the literature has discussed the stratification of the tumor microenvironment (TME) and classified it into four types. Of these, it has been reported that type I TME contains PD-1-expressing tumor-specific CD8+ T cells which is in close proximity to PD-L1-expressing cells. These types of tumors with a greater mutation load have been found to be more sensitive to anti-PD-1/PD-L1 therapy [41]. A separate study has also shown that mismatch repair status predicted the clinical benefit of anti-PD-1 therapy [42]. Therefore, accumulating evidence suggests that PD-L1 is expressed at higher levels in the EBV and MSI subgroups, likely due to viral stimulation and elevated mutational load. As such, these represent promising subtypes for the use of PD-1/PD-L1 blockade therapy, as these patients are most likely to benefit from immune inhibitory checkpoint blockade therapy.
9.2.2.3 Clinical Studies of PD-1/PD-L1 Blockade in Gastric Cancer
A growing number of clinical projects aimed at evaluating the use of anti-PD-1 and anti-PD-L1 immune checkpoint inhibitors for advanced gastric cancer are currently recruiting or ongoing. For instance, the KEYNOTE-012 trial (NCT01848834) enrolled 162 gastric cancer patients for screening. Among them, 65 patients were PD-L1 positive (40%). A total of 39 patients were enrolled in the trial and treated bi-weekly with pembrolizumab (10 mg/kg). The objective response rate (ORR) was 22%, and the clinical response was associated with PD-L1 expression [43, 44]. The most common treatment-associated adverse events (AEs) were hypothyroidism (n = 5) and fatigue (n = 5). Grade ≥ 3 AEs were observed in three patients, who were treated for hypoxia, peripheral neuropathy, and pneumonitis, respectively. This trial provided the first evidence for the feasibility of clinical application of the PD-1 blocking antibody for use in treating gastric cancer. However, the application of immune checkpoint inhibitors for gastric cancer is still in its infancy, and a growing number of clinical trials targeting PD-1 and PD-L1 are ongoing. It should be noted that there have also been studies of PD-L/PD-L1 blockade in combination with traditional therapy or other immune checkpoint inhibition for the treatment of gastric cancer (Table 9.3). It is unknown whether those patients who responded well belonged to the EBV-positive or MSI-positive subgroups. As such, further investigation into the molecular pattern of patients who could benefit most from the PD-1/PD-L1 blockade will be needed.
Table 9.3
Clinical trials for PD-1/PD-L1 inhibition in gastric cancer
Study | Subject | Agent | Stage | Status |
|---|---|---|---|---|
NCT02267343 | Study in patients with unresectable advanced or recurrent gastric cancer | Nivolumab | Phase III | Recruiting |
NCT02335411 | Pembrolizumab as monotherapy and in combination with cisplatin + 5-fluorouracil with recurrent or metastatic gastric or gastroesophageal junction adenocarcinoma (KEYNOTE-059) | Pembrolizumab | Phase I/IIA | Recruiting |
NCT02268825 | Study of MK-3475 with chemotherapy in patients with advanced GI cancers (MK-3475 GI) | Pembrolizumab | Phase I/II | Recruiting |
NCT02340975 | Study of MEDI4736 with tremelimumab, MEDI4736, or tremelimumab monotherapy in gastric or GEJ adenocarcinoma | MEDI4736 tremelimumab | Phase I/II | Recruiting |
NCT02625610 | Avelumab in first-line gastric cancer | Avelumab | Phase III | Recruiting |
NCT01848834 | Study of pembrolizumab in participants with advanced solid tumors (KEYNOTE-012) | Pembrolizumab | Phase I | Ongoing |
NCT02625623 | Avelumab in third-line gastric cancer (JAVELIN Gastric 300) | Avelumab | Phase III | Recruiting |
NCT02268825 | Study of MK-3475 with chemotherapy in patients with advanced GI cancers | Pembrolizumab | Phase I/II | Recruiting |
NCT02589496 | Study of pembrolizumab with advanced gastric or gastroesophageal junction adenocarcinoma progressed after first-line therapy | Pembrolizumab | Phase II | Recruiting |
NCT02340975 | Study of MEDI4736 with tremelimumab, MEDI4736, or tremelimumab monotherapy in gastric or GEJ adenocarcinoma | MEDI4736 tremelimumab | Phase I/II | Recruiting |
NCT02494583 | Study of pembrolizumab as first-line monotherapy and combination therapy for advanced gastric or gastroesophageal junction adenocarcinoma (KEYNOTE-062) | Pembrolizumab | Phase III | Recruiting |
NCT02370498 | Study of pembrolizumab versus paclitaxel for participants with advanced gastric/gastroesophageal junction adenocarcinoma progressed after first-line therapy (KEYNOTE-061)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|