Since the development and approval of Ipilimumab, the first immune checkpoint inhibitor licensed for the treatment of metastatic melanoma, clinicians have gained a better understanding of the mode of action, management of toxicities, and assessment of response to this class of drugs. Several antibodies are now in development, aimed at blocking novel immune checkpoint molecules, such as PD-1 and it’s corresponding ligand PD-L1. This article summarizes the mechanism of action, preclinical development, and subsequent clinical studies of immune checkpoint antibodies in melanoma.
Key points
- •
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) are immune checkpoint molecules. These co-inhibitory molecules on the surface of T-cells and self-cells regulate T-cell function by shutting down an immune stimulus.
- •
Ipilimumab is an anti-CTLA4 antibody that reinvigorates an antitumor T-cell response. It is a therapy approved by the Food and Drug Administration for first-line treatment of metastatic melanoma.
- •
Nivolumab and MK-3475 are anti–PD-1 antibodies, and MPDL3280A is an antibody that blocks the ligand of PD-1, PD-L1. These agents have demonstrated promising results in the treatment of advanced melanoma in early-phase studies.
- •
Immune-related response criteria are a novel set of radiologic criteria used to define response to immunotherapeutic agents.
- •
Immune-related adverse events are side effects of immunotherapeutic agents associated with cytokine release and immune infiltration of organs.
Introduction
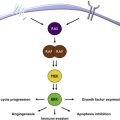
The immune system performs the vital function of defense against foreign antigens. In doing so, it requires a variety of checks and balances to protect against self-antigens while assuring appropriate activation against foreign antigens. These checks and balances consist of immune-activating and inhibiting receptors and ligands expressed by T-cells and other immune cell subsets. Antigen recognition by T-cells occurs through the engagement of the T-cell receptor (TCR) and peptide major histocompatibility complexes (MHC), as well as co-stimulation through CD28 binding to either B7-1 (CD80) or B7-2 (CD86) on antigen-presenting cells (APCs). Several of these molecules also play a role in T-cell activity in chronic infections and in cancer. CD28 is thus the first checkpoint in T-cell activation, as it responds to the expression of CD80/CD86 on an APC. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a co-inhibitory molecule that acts at this checkpoint. CTLA-4 binds with high affinity to CD80/CD86, and can block T-cell activation by direct competition with CD80/CD86 and by downstream CTLA-4–mediated inhibitory signaling.
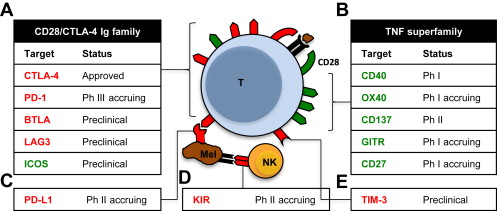
Immune checkpoints thus refer to a group of molecules on the surface of both self-cells and cells of the immune system that send a co-inhibitory stimulus, and in so doing attenuate an immune response ( Fig. 1 ). Therefore, monoclonal antibodies that bind and block the inhibitory actions of CTLA-4 might promote antigen-mediated T-cell activation. Melanoma is a cancer type with known immunogenic properties. The T-cell stimulatory cytokine interleukin-2 is already a Food and Drug Administration (FDA)-approved drug licensed for the treatment of this disease, and has been known to produce rare but durable long-term responses in both melanoma and renal cell carcinoma (RCC). These diseases thus provide the first ports of call for the development of further immunotherapies. Examples of immune checkpoints with potential therapeutic benefit include: CTLA-4, programmed death 1 (PD-1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin protein 3 (TIM-3), as detailed in Fig. 1 .
Introduction
The immune system performs the vital function of defense against foreign antigens. In doing so, it requires a variety of checks and balances to protect against self-antigens while assuring appropriate activation against foreign antigens. These checks and balances consist of immune-activating and inhibiting receptors and ligands expressed by T-cells and other immune cell subsets. Antigen recognition by T-cells occurs through the engagement of the T-cell receptor (TCR) and peptide major histocompatibility complexes (MHC), as well as co-stimulation through CD28 binding to either B7-1 (CD80) or B7-2 (CD86) on antigen-presenting cells (APCs). Several of these molecules also play a role in T-cell activity in chronic infections and in cancer. CD28 is thus the first checkpoint in T-cell activation, as it responds to the expression of CD80/CD86 on an APC. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a co-inhibitory molecule that acts at this checkpoint. CTLA-4 binds with high affinity to CD80/CD86, and can block T-cell activation by direct competition with CD80/CD86 and by downstream CTLA-4–mediated inhibitory signaling.
Immune checkpoints thus refer to a group of molecules on the surface of both self-cells and cells of the immune system that send a co-inhibitory stimulus, and in so doing attenuate an immune response ( Fig. 1 ). Therefore, monoclonal antibodies that bind and block the inhibitory actions of CTLA-4 might promote antigen-mediated T-cell activation. Melanoma is a cancer type with known immunogenic properties. The T-cell stimulatory cytokine interleukin-2 is already a Food and Drug Administration (FDA)-approved drug licensed for the treatment of this disease, and has been known to produce rare but durable long-term responses in both melanoma and renal cell carcinoma (RCC). These diseases thus provide the first ports of call for the development of further immunotherapies. Examples of immune checkpoints with potential therapeutic benefit include: CTLA-4, programmed death 1 (PD-1), lymphocyte activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin protein 3 (TIM-3), as detailed in Fig. 1 .
CTLA-4 inhibitors
The most well-known and widely studied immune checkpoint is CTLA-4.
Preclinical Studies with CTLA-4
CTLA-4 is a molecule expressed on the surface of CD-4 and CD-8 T-cells, and is a member of the CD28/immunoglobulin superfamily (IGSF). CTLA-4 competes with CD28 to bind to ligands B7-1 (CD80) and B7-2 (CD86) on APCs. CTLA-4 mediates an inhibitory signal, thereby dampening T-cell responses in the tumor microenvironment (TME). CTLA-4 is also expressed on CD25 + FOXP3 + T-regulatory cells (Tregs), and plays a pivotal role in the function of Tregs and in the CD4:CD8 ratio in the TME. CTLA-4 blockade has also been shown to deplete intratumoral Tregs. CTLA4 knockout mice demonstrate an increase in activated T-cells after 3 to 4 weeks, which manifests clinically as pancreatitis, myocarditis, and T-cell infiltration of the liver, heart, and lungs of the mice. When CTLA-4 signaling is blocked, protein tyrosine kinases FYN, LCK, and ZAP-70 are activated. To control T-cell activation CTLA-4 recruits 2 phosphatases, SHP2 and PP2A, which control these protein tyrosine kinases. CTLA-4 activation of SHP2 results in dephosphorylation of the CD3z chain, dampening the signal from the TCR. CTLA-4 recruitment of PP2A inhibits Akt phosphorylation, further dampening the TCR signal.
Blocking the action of CTLA-4 with an anti–CTLA-4 antibody was first studied in transplantable tumor models of colon carcinoma (51BLim10), fibrosarcoma (Sa1 N and CSA1 M), ovarian cancer (OV-HM), and prostate cancer (TRAMPC1). In these models, the blocking of CTLA-4 caused primary shrinkage of tumors and protected the mice against tumor rechallenge. Thus it was initially postulated that blocking CTLA-4 could induce durable control of tumors in humans.
Ipilimumab
Ipilimumab is a fully humanized immunoglobulin (Ig)G1 monoclonal antibody that binds to and inhibits the action of CTLA-4. In an initial trial of 17 patients with unresectable melanoma treated with ipilimumab, there were 2 partial responses (PRs) at the starting dose of 3 mg/kg administered as a single dose. These responses were durable, with the only notable adverse event (AE) being a mild rash. Ipilimumab was then combined with a glycoprotein (gp)100 peptide vaccine in a phase I clinical trial, where 14 patients with advanced melanoma were treated with 3 mg/kg of ipilimumab followed by a gp100 peptide vaccine. Three patients responded to treatment, including 2 complete responses (CRs). However, more than 3 subjects experienced grade 3 and 4 AEs that appeared to be immune-related, including dermatitis, enterocolitis, hypophysitis, and hepatitis. A dose-response relationship was demonstrated in a double-blind phase II trial of ipilimumab monotherapy administered at 3 dose levels in 217 patients with advanced melanoma. These patients received 0.3, 3, or 10 mg/kg of antibody every 3 weeks for 4 doses. If patients experienced stable disease (SD) or a response to therapy, and tolerated treatment, they subsequently received maintenance 12-weekly ipilimumab. A response rate (RR) of 11% and a median overall survival (OS) of 14 months were reported in the 10 mg/kg group. This dose is thus considered to be the optimum one, although it is also associated with the greatest rate of immune-related (ir) AEs.
Ipilimumab is now an FDA-approved therapy for metastatic melanoma (Yervoy; Bristol Myers-Squibb, New York, NY). FDA approval of ipilimumab was based on the results of 2 phase III randomized controlled trials (RCTs) that demonstrated an OS benefit with ipilimumab. A phase III trial of pretreated patients with advanced melanoma was a 3-arm study that compared ipilimumab at a dose of 3 mg/kg with or without gp100 peptide vaccine, with the gp100 peptide vaccine alone. The median OS in the ipilimumab and ipilimumab/gp100 groups was 10.1 versus 10.0 months, respectively, compared with 6.4 months with gp100 alone (hazard ratio [HR] 0.68, P <.001). The first-line phase III RCT compared ipilimumab/dacarbazine with dacarbazine/placebo, at an ipilimumab dose of 10 mg/kg, followed by maintenance ipilimumab or placebo every 12 weeks. This trial demonstrated an improvement in median OS in the combination arm (11.2 vs 9.1 months). Of note, higher survival rates were seen in the ipilimumab/dacarbazine group at 1 year (47.3% vs 36.3%), 2 years (28.5% vs 17.9%), and 3 years (20.8% vs 12.2%) (HR for death 0.72; P <.001). The median duration of response was 19.3 months with the combination and 8.1 months with dacarbazine alone. AEs of all grades were more common in the combination arm, most notably in elevations of levels of alanine aminotransferase (ALT) (33.2% vs 5.6%) and aspartate aminotransferase (AST) (29.1% vs 5.6%), diarrhea (36.4% vs 24.7%), pruritus (29.6% vs 8.8%), and rash (24.7% vs 6.8%). Grade 3 or 4 AEs were noted in 56.3% of patients in the combination arm, compared with 27.5% of those receiving dacarbazine/placebo ( P <.001).
The potential benefit of reinduction ipilimumab has also not been fully elucidated. In the ipilimumab/gp100 trial reported by Hodi and colleagues, 31 patients were reinduced. Nineteen percent (n = 6/31) of patients demonstrated a treatment response, and 48% (n = 15/31) had SD. The benefit of reinduction ipilimumab will be investigated in a trial comparing this agent with investigator’s choice of chemotherapy ( NCT00495066 ).
Tremelimumab
Tremelimumab is an inhibitory human IgG2 monoclonal antibody against CTLA-4. Tremelimumab has been investigated in 9 clinical studies as a single agent, with a total of more than 1000 patients having received this agent. Early-phase studies with tremelimumab demonstrated a 6% to 10% RR, with durable responses lasting for more than 170 days. Tremelimumab was compared with investigator’s choice chemotherapy in a phase III study in advanced melanoma, at a dose of 15 mg/kg every 3 months. This study demonstrated a median duration of response of 35.8 months, compared with 13.7 months with combination chemotherapy ( P = .0011), but did not demonstrate an OS benefit. This finding can potentially be explained by the exclusion of patients with an elevated level of lactate dehydrogenase onto this study, crossover of patients to ipilimumab, and the possibility of suboptimal dose and schedule.
Adverse Events
A spectrum of AEs associated with CTLA-4 inhibitors are well described, and are termed irAEs. irAEs relate to the mode of action of these agents, which can lead to the development of a CD-4 and CD-8 T-cell inflammatory infiltration of solid organs, and increased serum inflammatory cytokines. irAEs can occur at any time point during ipilimumab therapy and can range in severity from mild to fatal, and in onset from slow to sudden. Management is aimed at correctly identifying the irAE, grading the toxicity based on National Cancer Institute common toxicity criteria of AEs, and initiating early treatment with supportive care or steroid medications. The incidence of grade 3 or higher irAEs in published studies ranges from 5% to 25%, and is dose related. The most common irAEs are dermatitis (pruritus, rash), enterocolitis, endocrinopathies (hypophysitis, thyroiditis), liver abnormalities (elevated serum liver tests, hepatitis), and uveitis. In the trial reported by Hodi and colleagues, the most frequent AEs were rash (30%), pruritus (33%), diarrhea (33%), colitis (8%), endocrine abnormalities (9%), AST/ALT elevations (2%), and hepatitis (1%). irAEs with tremelimumab in the study reported by Ribas and colleagues were similar to those of ipilimumab, such as colitis, rash, vitiligo, hepatitis, and autoimmune endocrinopathies.
Management of irAEs
Management algorithms have emerged for irAEs such as diarrhea/colitis. Grade I diarrhea is managed with oral hydration, an American Dietary Association colitis diet, and loperamide. Diarrhea of grade 2 or greater can be managed with oral budesonide, oral steroids, intravenous methylprednisolone, and, occasionally, infliximab. In a post hoc analysis of patients with colitis of grade 3 or higher, 90% of patients received corticosteroids, and 14% of these patients received further immunosuppression with infliximab. Median time until resolution of diarrhea was 2 weeks. Colitis-associated mortality was associated with management delays, failure to withhold ipilimumab, and an inadequate antidiarrheal regimen. In a series of 15 reported cases of ipilimumab-related colitis requiring infliximab, treatment was effective within 3 days in most subjects. Administration of corticosteroids has not been associated with changes in survival or duration of response to anti–CTLA-4 therapy. The use of effective management algorithms have reduced life-threatening complications, with bowel perforations occurring in fewer than 1% of patients ( Table 1 ).
| Agent | Study Design | No. of Patients | Response | Survival (mo) | Grade 3/4 Adverse Events |
|---|---|---|---|---|---|
| Ipilimumab | Phase III Ipilumumab + gp100 vs gp100 | 676 | 6% ORR 14% SD (ipi + gp100) | 6.0 vs 10.0 | Overall (ipi + gp100) 17% Colitis 1% Diarrhea 5% Endocrinopathy 1% Abnormal LFTs 1% |
| Phase III Ipilimumab + dacarbazine vs dacarbazine | 502 | — | 11.2 vs 9.1 | Overall (ipi + dacarb) 42% High ALT 21% High AST 17% Diarrhea/colitis 6% Pruritus 2% | |
| Tremelimumab | Phase III Treme vs physician’s choice chemotherapy | 655 | 10.7% (treme) 9.8% (chemo) | 12.6 vs 10.7 | Overall 52% Diarrhea 15% Fatigue 6% Nausea 4% Vomiting 4% |
| Phase II Treme + pegIFN α2b | 37 | 24% ORR 38% SD | 21.0 overall survival | Neutropenia 16% Diarrhea 11% Abnormal LFTs 11% |
Evaluating Response with CTLA-4 Inhibitors
When analyzing the Kaplan-Meier survival curves of the 2 phase III studies reported by the groups of Hodi and Robert, a characteristic shape of the survival curves is noted. First, the survival curves overlap until approximately 4 months, after which the ipilimumab arm diverges, and subsequently plateaus. This pattern indicates that a subset of patients achieves a benefit, and that this benefit is durable. Long-term follow-up of patients treated on earlier-phase studies with ipilimumab confirms durable responses, with 4-year OS ranging from 13.8% to 49.5% at various doses of ipilimumab. It is with this in mind that Wolchok and colleagues first described the immune-related response criteria (irRC) tailored toward evaluating response to immunotherapeutic agents. These criteria are based on the rationale that immunotherapies generate an antitumor effect with response kinetics distinct from those of cytotoxic chemotherapy. Antitumor responses with immunotherapeutic agents may be delayed; in some patients, lesions may enlarge before ultimately shrinking, perhaps related to the underlying immune mechanism of T-cell activation and tumoral infiltration. Patients may also achieve SD with slow tumor regression over time. The irRC thus recommend interval imaging at least 4 weeks apart to aid in the confirmation of progression. In determining SD or PR, new lesions are allowed in the context of overall decreases in tumor burden. In contrast to traditional World Health Organization criteria, new non-measurable lesions do not define progression in irRC criteria. Because of these response patterns, patients who experience a clinically insignificant progression of disease often continue to receive therapy until progression is confirmed on subsequent imaging.
Anti–PD-1 antibodies
Preclinical Studies
PD-1 (CD279) is a transmembrane molecule on T lymphocytes, B lymphocytes, and monocytes that acts as an immune checkpoint. When PD-1 is stimulated, the immune response mediated by these cells is inhibited. The PD-1 signaling pathway is mediated by the binding of PD-1 to ligands PD-L1 and PD-L2 on APCs, as well as PD-L1 binding to the co-stimulatory molecule B7-1. The term “programmed death” was given to this molecule on its identification in 1992 as a gene upregulated in T-cell hybridoma undergoing cell death. The structure of PD-1 consists of an IGSF domain, a transmembrane domain, an intracellular domain containing an immunoreceptor tyrosine-based inhibitory motif, and an immunoreceptor tyrosine-based switch motif (ITSM). The inhibitory function of PD-1 is lost when the ITSM is mutated, suggesting that this tyrosine plays a primary functional role. During PD-1 pathway activation, PD-1 can recruit phosphatases SHP-1 and SHP-2, and causes dephosphorylation of the CD3z chain. PD-1 and CTLA-4 therefore mediate their effects through slightly different mechanisms, as PD-1 inhibits Akt activation via the phosphoinositide 3-kinase (PI3K) pathway, whereas CTLA-4 inhibits Akt independent of PI3K. PD-1 knockout mice demonstrate elevated serum IgG2b and IgA levels, and develop a lupus-like syndrome and dilated cardiomyopathy. These side effects appear less frequently and later in life in PD-1 knockout mice than in CTLA-4 knockout mice.
Nivolumab
Nivolumab (BMS-936558; anti–PD-1 monoclonal antibody [mAb]) is a fully human monoclonal IgG4 antibody that binds to the PD-1 cell-surface membrane receptor. Sosman and colleagues initially investigated nivolumab in a phase I study of 107 patients with metastatic melanoma. Patients received doses from 0.1 to 10 mg/kg, and demonstrated an overall response rate (ORR) of 31%, with an RR of 41% in the 3 mg/kg group. Excellent durability of responses was seen, with 61% 1-year and 44% 2-year survival rates. Treated-related AEs included fatigue, rash, diarrhea, and pruritus. Grade 3 and 4 toxicities were reported in 21% of patients, with an incidence of 1% to 3% in fatigue, diarrhea, nausea, and anemia. The antitumor effect of PD-1 receptor blockade by nivolumab has been investigated in several tumor types. Results from a phase I/II study reported by Topalian and colleagues indicate that nivolumab is active in multiple tumor types. Treatment-related pneumonitis was reported in 3% (n = 9) of these patients, and accounted for 3 patient deaths in this study, none of which had melanoma. Nivolumab monotherapy at a dose of 3 mg/kg is currently being studied in phase III clinical trials in advanced melanoma, RCC, and non–small lung carcinoma (NSCLC). In melanoma, a first-line study of the 3 mg/kg dose of nivolumab is currently being studied in a comparison with dacarbazine (NCT01721772). Successful reinduction therapy with nivolumab has been described in a patient with melanoma who achieved a PR, followed by a period of SD for 16 months off treatment. Reinduction resulted in a successful response after relapse in this patient.
MK-3475
MK-3475 is a humanized monoclonal IgG4 PD-1 antibody. A phase I dose-escalation study involving patients with multiple solid tumor types demonstrated the safety of this agent at the dose levels 1 mg/kg, 3 mg/kg, and 10 mg/kg administered every 2 weeks, and no maximum tolerated dose was identified. In addition, clinical responses were observed at all the dose levels. MK-3475 was studied as a single agent in patients with advanced melanoma, in both patients who had previously received ipilimumab and those who had not. A phase I study reported by Hamid and colleagues tested 2 doses, 2 and 10 mg/kg, given every 2 or 3 weeks. The immune-related responses in patients who had and had not received prior ipilimumab were identical, at 56%, in patients who received the 10 mg/kg dose every 2 weeks. Responses were 22% and 33% in patients who received the 3-weekly 10 mg/kg dose with and without prior ipilimumab, respectively. Seventy-nine percent (n = 107) of patients reported treatment-related AEs of all grades, and 13% reported grade 3 or 4 AEs. Pneumonitis occurred in 4% of patients, with no cases of grade 3 or 4 events. A phase II trial of this agent at 2 dose levels in comparison with chemotherapy is currently in accrual (NCT01704287.) The large experience with MK-3475 reports RRs close to 50%, and, again, excellent response durability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree