Immediate Hypersensitivity: Approach to Diagnosis
Nkiruka U. Erekosima
Sarbjit S. Saini
I. BACKGROUND: HISTORICAL PERSPECTIVE OF IMMUNOGLOBULIN E
Nearly a century ago, Portier and Richet reported a paradoxical immunologic effect when trying to enhance the resistance of dogs to sea anemone toxin by injecting the dogs with the toxin. Rather than conferring the expected protection, when reinjected weeks later with minute amounts of the same toxin, several animals died within minutes of cardiorespiratory collapse. To describe this phenomenon, they coined the term anaphylaxis (meaning antiprotection). Subsequent studies determined that these reactions required prior exposure to the foreign substance and a period of weeks to manifest the response. They also found that anaphylactic sensitivity could be transferred from one animal to another by injecting or infusing a serum-derived factor, initially referred to as reagin. Soon thereafter, studies in humans revealed that injection of serum from an anaphylactically sensitive individual into a nonsensitive individual (passive transfer) led to local sensitization (transfer of a wheal-and-flare reaction) in the recipient at the cutaneous site. The passive transfer experiment, first performed by Prausnitz and Kustner, and now referred to as the PK test or reaction, gave the first indication that allergic hypersensitivity resulted from a serum protein. This type of response became known as immediate hypersensitivity due to the rapidity of the reaction. However, the composition of reagin remained a mystery until the 1960s, when Ishizaka and Ishizaka purified and identified immunoglobulin E (IgE), establishing that reagin was an antibody molecule. It is now clear that the presence of specific IgE antibodies represents the single most important determinant of allergic sensitivity. Synthesis or passive transfer of IgE antibodies sensitizes the recipient for both the immediate allergic response and any subsequent reactions. Today, the term allergen refers to a protein, typically a common innocuous antigen that elicits IgE antibody production. Allergy refers to the clinical manifestations of IgE-dependent immunologic reactions, whereas atopy is used to describe the genetic tendency to generate IgE responses.
II. ANTIGEN CHARACTERISTICS
Antigens. For a substance to generate an immune response, it must be presented to the immune system in an appropriate fashion. Characteristics of the antigen can determine whether the immune system develops an antibody response of a particular class or whether an antibody response is generated at all. For example, so-called T-cell-independent antigens such as polysaccharides do not typically give rise to IgE antibodies. Thus, the nature of the antigen is an important
determinant of the host response. Although a complete understanding of what makes an antigen an allergen has not been achieved, several general principles can be stated. Most common allergens (e.g., pollen, dust mites, animal dander) that cause airway symptoms in humans are proteins with a molecular weight of 10 to 20 kDa, are highly water-soluble, and can act as complete allergens. Repeated, low-level exposure to these proteins (typically in the submicrogram range) diffusing across mucosal surfaces is particularly efficient at inducing an IgE response. Host characteristics are also key in generating IgE responses, given that about 20% of the exposed population, that is, those with the atopic trait, will generate IgE antibodies. Some of the most prevalent aeroallergens, such as dust mites (Der p 1, a cysteine protease), have enzymatic functions in their natural state. However, a clear association between the functional properties of an allergen and its immunogenicity, that is, the ability to generate immunologic responses in susceptible subjects, is lacking. It has also been difficult to identify common structural features among known allergenic proteins to allow predictions of the immunogenicity of novel peptides. However, the cross-reactivity of a protein with preexisting IgE antibodies to another allergen has been shown to be based on structural similarities and presents clinically, for example, in the pollen-food allergy syndrome. Here, the ingestion of labile proteins found in, for instance, fresh apples, can cross-react with an individual’s existing birch pollen-specific IgE, leading to the occurrence of itching of the oral mucosa. Cooking the apple rapidly inactivates the cross-reactive allergen, thus eliminating the symptoms.
determinant of the host response. Although a complete understanding of what makes an antigen an allergen has not been achieved, several general principles can be stated. Most common allergens (e.g., pollen, dust mites, animal dander) that cause airway symptoms in humans are proteins with a molecular weight of 10 to 20 kDa, are highly water-soluble, and can act as complete allergens. Repeated, low-level exposure to these proteins (typically in the submicrogram range) diffusing across mucosal surfaces is particularly efficient at inducing an IgE response. Host characteristics are also key in generating IgE responses, given that about 20% of the exposed population, that is, those with the atopic trait, will generate IgE antibodies. Some of the most prevalent aeroallergens, such as dust mites (Der p 1, a cysteine protease), have enzymatic functions in their natural state. However, a clear association between the functional properties of an allergen and its immunogenicity, that is, the ability to generate immunologic responses in susceptible subjects, is lacking. It has also been difficult to identify common structural features among known allergenic proteins to allow predictions of the immunogenicity of novel peptides. However, the cross-reactivity of a protein with preexisting IgE antibodies to another allergen has been shown to be based on structural similarities and presents clinically, for example, in the pollen-food allergy syndrome. Here, the ingestion of labile proteins found in, for instance, fresh apples, can cross-react with an individual’s existing birch pollen-specific IgE, leading to the occurrence of itching of the oral mucosa. Cooking the apple rapidly inactivates the cross-reactive allergen, thus eliminating the symptoms.
A. Complete protein allergens. By definition, proteins that are complete allergens have (i) the property to induce the production of IgE antibodies; (ii) the ability to elicit an allergic reaction or trigger symptoms in a sensitive host; and (iii) the property to bind IgE antibodies with sufficient numbers of antigenic determinants.
B. Incomplete protein allergens. An incomplete allergen is one that is able to elicit symptoms in a sensitive host but cannot independently generate an IgE antibody response. Commonly, these are low-molecular-weight drugs such as the β-lactam family of antibiotics. On the basis of studies with penicillin, the β-lactam molecule generates reactive metabolites in vivo that covalently bind to a number of the host’s normal proteins, such as albumin. These reactive compounds are termed haptens, and by definition they must bind to other proteins (haptenate) in vivo to elicit an IgE response. Penicillin hapten-protein complexed allergens, also called major and minor determinants, have been identified and used for diagnostic skin testing. However, it has been difficult to establish standard skin testing reagents for the evaluation of other incomplete drug allergens, given the multiple pathways to generate drug intermediates and their potential for haptenization.
III. PRODUCTION OF IMMUNOGLOBULIN E
If many individuals are exposed to the same antigen by the same route, only a few develop IgE antibodies, and therefore only a minority of these people are at risk for allergic reactions upon reexposure. There is also the concept of asymptomatic sensitization; for example, 8% of the US population has a positive skin test to peanut, but <1% have clinical manifestations of peanut allergy. Why only a minority of people make antigen-specific IgE and become allergic remains
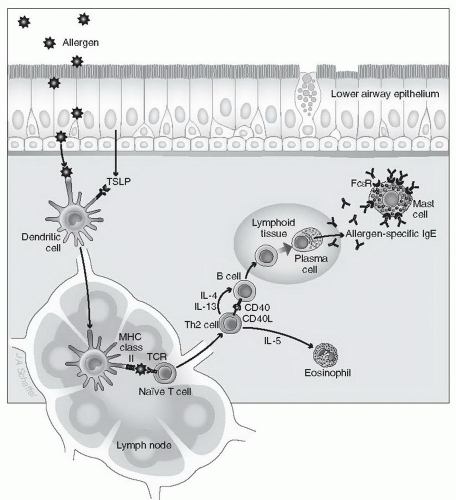
unknown. If such events are to occur, however, a distinct series of immunologic events must happen (Fig. 2-1). First, an allergen needs to cross an epithelial barrier. The epithelium can provide signals that favor allergic sensitization. The allergen is next internalized by antigen-presenting cells, such as dendritic cells (DCs), and, after processing, is presented to T lymphocytes. Recent studies have demonstrated that thymic stromal lymphopoietin (TSLP), an epithelial cell-derived cytokine, has an important role in initiating allergic inflammation (see Chapter 1, Section III, D), by polarizing DCs to drive T helper (Th2) cell inflammation. If an IgE antibody response is to occur, these events must take place in the presence of specific cytokines, the most important of which is interleukin (IL)-4 released by T lymphocytes and other cells. IL-4 is critical for the generation of the T lymphocytes (Th2 cells) themselves and for subsequent stimulation by these Th2 cells of B lymphocytes. If this stimulation occurs in the presence of IL-4 and IL-13, the B cells undergo immunoglobulin gene class switching, leading to their terminal differentiation into plasma cells that produce antigen-specific IgE antibodies. Generation of IgE-producing plasma cells is also facilitated by binding of CD40 ligand on the Th2 cell to CD40 on the B cell. Once plasma cells undergo these steps, they release the exact same antigenspecific IgE for the rest of their lives. This IgE secretion by plasma cells typically takes place at mucosal sites, but it can also occur in lymph nodes and other lymphoid organs. Among immunoglobulins, IgE has several unique features. For example, unlike IgG, IgE cannot cross the placenta, and it does not bind complement. IgE contains an additional region in its heavy chain that makes the molecule uniquely capable of binding to specific IgE receptors. IgE is also heavily glycosylated, although the biologic significance of this is uncertain. Finally, although the presence of IgE correlates with allergic diseases, high levels of IgE are seen in other disorders, such as helminthic parasite infections, whereas low levels of serum IgE overlap between allergic and nonallergic persons.
unknown. If such events are to occur, however, a distinct series of immunologic events must happen (Fig. 2-1). First, an allergen needs to cross an epithelial barrier. The epithelium can provide signals that favor allergic sensitization. The allergen is next internalized by antigen-presenting cells, such as dendritic cells (DCs), and, after processing, is presented to T lymphocytes. Recent studies have demonstrated that thymic stromal lymphopoietin (TSLP), an epithelial cell-derived cytokine, has an important role in initiating allergic inflammation (see Chapter 1, Section III, D), by polarizing DCs to drive T helper (Th2) cell inflammation. If an IgE antibody response is to occur, these events must take place in the presence of specific cytokines, the most important of which is interleukin (IL)-4 released by T lymphocytes and other cells. IL-4 is critical for the generation of the T lymphocytes (Th2 cells) themselves and for subsequent stimulation by these Th2 cells of B lymphocytes. If this stimulation occurs in the presence of IL-4 and IL-13, the B cells undergo immunoglobulin gene class switching, leading to their terminal differentiation into plasma cells that produce antigen-specific IgE antibodies. Generation of IgE-producing plasma cells is also facilitated by binding of CD40 ligand on the Th2 cell to CD40 on the B cell. Once plasma cells undergo these steps, they release the exact same antigenspecific IgE for the rest of their lives. This IgE secretion by plasma cells typically takes place at mucosal sites, but it can also occur in lymph nodes and other lymphoid organs. Among immunoglobulins, IgE has several unique features. For example, unlike IgG, IgE cannot cross the placenta, and it does not bind complement. IgE contains an additional region in its heavy chain that makes the molecule uniquely capable of binding to specific IgE receptors. IgE is also heavily glycosylated, although the biologic significance of this is uncertain. Finally, although the presence of IgE correlates with allergic diseases, high levels of IgE are seen in other disorders, such as helminthic parasite infections, whereas low levels of serum IgE overlap between allergic and nonallergic persons.
IV. IgE RECEPTORS
Secreted IgE produced by allergen-specific B cells binds to specialized Fc receptors on the surface of mast cells and basophils throughout the body. Although free IgE has a short serum half-life (2 to 3 days compared to 21 days for IgG), IgE bound to the surface of mast cells can persist for several months. The high-affinity IgE receptor (FcεRI) expressed by human mast cells and basophils is a tetramer composed of one alpha, one beta, and two gamma chains. Despite the low serum concentration of IgE (typically in the nanogram per milliliter range), most surface FcεRI receptors are occupied, given that the dissociation constant is 1 × 10-10 M. The alpha chain binds to IgE at its third heavy chain constant domain, whereas the β and γ subunits of the receptor are involved in transducing signals generated by activation of the receptor complex (Fig. 2-2). The therapeutic anti-IgE antibody, omalizumab, designed for clinical use in allergic disease, takes advantage of this fact by binding to the third heavy chain of free IgE, competitively inhibiting IgE from binding to the alpha chain of FcεRI. The overall number of high-affinity receptor complexes on basophils ranges from a few thousand to 1 million molecules per cell and is positively related to the level of circulating free IgE as well as to levels of expression of the beta subunit. Both IgE levels and cytokines such as IL-4 and IL-9 are believed to enhance mast cell
FcεRI expression. An alternate, trimeric high-affinity IgE receptor (composed of one α and two γ chains) occurs on monocytes, DCs, and Langerhans’ cells, especially in allergic hosts; these cells utilize this receptor to capture antigen for eventual presentation by these cells to T cells. Dc IgE receptor-facilitated allergen presentation to T cells can be downregulated by omalizumab.
FcεRI expression. An alternate, trimeric high-affinity IgE receptor (composed of one α and two γ chains) occurs on monocytes, DCs, and Langerhans’ cells, especially in allergic hosts; these cells utilize this receptor to capture antigen for eventual presentation by these cells to T cells. Dc IgE receptor-facilitated allergen presentation to T cells can be downregulated by omalizumab.
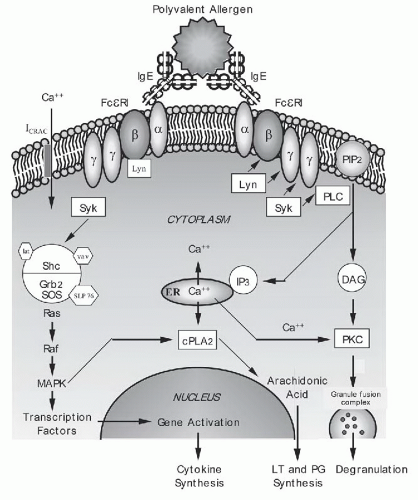 Figure 2-2. Cross-linking of immunoglobulin E (IgE) activates intracellular biochemical pathways. Once mast cells or basophils are sensitized by IgE binding to the FcεRI alpha chain, they can be activated by polyvalent allergen that cross-links adjacent receptors. See text on pages 36 and 37 for details. |
Another receptor for IgE, termed FcεRII (or CD23), has low affinity for binding IgE, with a single transmembrane chain that is unrelated to FcεRI. CD23 binds to the Cε3 domain of IgE, which is different from the FcεRI binding site. Expression of CD23 is upregulated by IgE and IL-4. It is expressed as two different forms: CD23a and CD23b. CD23a is expressed constitutively on B cells, and CD23b is expressed on several cells, including monocytes, eosinophils, DCs, Langerhans’ cells, and platelets. CD23b is also inducible on B cells and some T cells by IL-4 or IL-13 stimulation. Activation of CD23 on B cells leads to multiple functions, including B-cell differentiation, regulation of IgE synthesis, apoptosis, activation of monocytes, and antigen presentation. CD23 also binds to other ligands, including the adhesion molecules CD11b and
CD11c, and these interactions lead to other functions. CD23 can be cleaved by proteases to generate a soluble CD23 form, which is proinflammatory. In vitro studies of a monoclonal antibody that targets CD23, lumiliximab, demonstrate that lumiliximab leads to decreased Th2 responses and reduced IgE synthesis.
CD11c, and these interactions lead to other functions. CD23 can be cleaved by proteases to generate a soluble CD23 form, which is proinflammatory. In vitro studies of a monoclonal antibody that targets CD23, lumiliximab, demonstrate that lumiliximab leads to decreased Th2 responses and reduced IgE synthesis.
V. MEASUREMENT AND CLINICAL SIGNIFICANCE OF IgE
Measurement of allergen-specific IgE is determined by skin testing or by detection of allergen-specific IgE in serum (discussed further in Chapter 20, Diagnostic Immunology). Increased IgE levels are seen in individuals with atopic diseases. In general, individuals with atopic dermatitis tend to demonstrate higher IgE levels than those observed in other atopic conditions. Measurement of allergen-specific IgE in serum is particularly useful in specific clinical situations when skin testing cannot be performed. It can also be used to monitor disease activity in certain conditions such as food allergy. Total serum IgE level is also useful for monitoring disease activity and response to therapy in patients with allergic bronchopulmonary aspergillosis. Elevated IgE levels are also present in several nonatopic conditions, such as parasitic infections, human immunodeficiency virus (HIV), certain autoimmune/inflammatory disorders, malignancies, and some primary immunodeficiency diseases.
Evidence for the role of human IgE in allergic disease has long been recognized by the ability to transfer allergen sensitivity by serum IgE (reaginic reactivity). More recently, trials of the humanized monoclonal anti-IgE, omalizumab, which reduces free IgE levels in serum, have shown clinical benefits in subjects with allergic asthma, allergic rhinitis, airway allergen challenge models, and conditions of anaphylaxis (food and rush immunotherapy); however, omalizumab is approved by the U.S. Food and Drug Administration (FDA) only for patients with moderate to severe persistent allergic asthma.
VI. MAST CELLS AND BASOPHILS
A. Origin and distribution. Mast cells and basophils can be identified microscopically by the presence of cytoplasmic granules that contain acidic molecules that take up basic dyes and stain metachromatically. Both cell types also bear high-affinity IgE receptors. They both originate from CD34+ hematopoietic, pluripotent stem cells residing in the bone marrow but differ in their developmental stages. Undifferentiated mast cells migrate from the bone marrow to the connective tissues of mucosal and epithelial surfaces of the body. They subsequently mature and take up residence in close proximity to blood vessels, nerves, and body surfaces in contact with the external environment. Mast cells (and some basophils) also uniquely express high levels of c-kit, a receptor that binds the important growth factor, stem cell factor (SCF). The local tissue environment can provide SCF as well as influence mast cell mediator content, granule ultrastructure, and functionality, thus contributing to heterogeneity among mast cell populations (tryptase-positive or chymase/tryptase-positive cells). In contrast, basophils mature in the bone marrow under the influence of IL-3, differ from mast cells in bearing high levels of CD123+ expression (receptor for IL-3), and reside in the circulation as mature, nondividing cells. Activated basophils express high levels of IL-4, IL-13, and CD40 ligand. In the past, basophils were thought to be a form of circulating mast cell but are now
considered closer in lineage to eosinophils. Basophils can migrate to tissue sites in response to inflammatory stimuli, such as those generated during the late phase of an allergic response (Fig. 2-3). Seasonal allergen exposure can cause an increase in the number of circulating basophils and tissue mast cells. Local application of steroids, such as in the nose of rhinitis patients, will blunt the increase in mast cells that is normally observed during the pollen season.
considered closer in lineage to eosinophils. Basophils can migrate to tissue sites in response to inflammatory stimuli, such as those generated during the late phase of an allergic response (Fig. 2-3). Seasonal allergen exposure can cause an increase in the number of circulating basophils and tissue mast cells. Local application of steroids, such as in the nose of rhinitis patients, will blunt the increase in mast cells that is normally observed during the pollen season.
B. Pathways of mast cell and basophil mediator release. Allergen crosslinking of IgE bound to the surface of mast cells and basophils leads to cellular activation, degranulation, and inflammatory mediator release. In addition to allergen, experimental antibodies that can cross-link cell-bound IgE or FcεRI receptors can also activate mast cells and basophils. In either case (allergen or cross-linking antibodies), bridging of as few as 1% of the total surface FcεRI (about 300 to 500 receptors) can be sufficient to activate the cells.
A number of IgE-independent pathways for activation of mast cells and basophils also exist. Most of these depend on the influx of calcium for cellular activation. Examples of in vivo triggers are drugs such as opiates (for skin mast cells), aspirin, and other nonsteroidal anti-inflammatory drugs (NSAIDS), smooth muscle relaxants, and intravenous radiocontrast dyes. Examples of in vitro agents are calcium ionophore and compound 48/80 (the latter for mast cell activation). There are also numerous biologic inflammatory stimuli that can activate or potentiate mediator release, including histamine-releasing factors, products of complement activation (C3a, C5a), nerve-related peptides (tachykinins, nerve growth factor, calcitonin gene-related peptide), adenosine
triphosphate, IL-1, IL-3, and several chemokines. In addition, skin mast cells in certain subjects can be activated by a variety of physical stimuli (such as cold temperature, pressure, sunlight, heat, or exercise) to produce urticaria.
triphosphate, IL-1, IL-3, and several chemokines. In addition, skin mast cells in certain subjects can be activated by a variety of physical stimuli (such as cold temperature, pressure, sunlight, heat, or exercise) to produce urticaria.
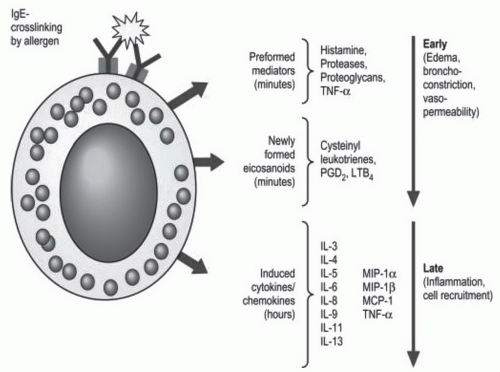 Figure 2-3. Mediators released from mast cells upon IgE-mediated activation. See description on pages 37 and 38 for details. (Reproduced from Hsu FI, Boyce JA. Biology of mast cells and their mediators. In: Adkinson NF, Bochner BS, Busse WW, et al., eds. Middleton’s allergy: principles and practice. Philadelphia, PA: Elsevier, 2009:311-328, with permission.) |
C. Releasability. The wide variation noted in the extent of histamine release from basophils of different individuals has been termed releasability. Factors that determine the sensitivity of basophils or mast cells to allergen activation, or the magnitude of their mediator release, are still not clear. There is some evidence that factors such as the ratio of allergen-specific IgE to total IgE, the affinity of IgE for the allergen, and levels of Syk (a tyrosine kinase necessary for proper signal transduction) influence releasability. About 5% to 10% of people have basophils that fail to release any histamine after IgE receptor cross-linking. These “non-releaser” basophils appear to be deficient in Syk (see Fig. 2-2). Allergen immunotherapy leads to a higher threshold for allergen-induced basophil mediator release and may be one of the mechanisms of action of this form of treatment. In cultured mast cell systems, the induction of greater numbers of FcεRI receptors by either IL-4 or IgE leads to increased sensitivity to allergen and increased magnitude of mediator responses, whereas treatment with omalizumab appears to counteract these effects. However, similar effects of increased FcεRI expression or surface-bound IgE on basophil mediator responses have not been observed.
D. Biochemical signaling pathways triggered by multivalent allergen. Antigen-induced FcεRI aggregation leads to the stimulation of a complex cascade of intracellular signaling events. Although these events have been extensively studied, the order of events and the exact molecules that participate in each cell type are not fully known. Figure 2-2 depicts a possible schema for FcεRI-related signaling events. The bridging of adjacent surface-bound IgE molecules by polyvalent allergen allows receptor-associated Src family kinase, Lyn, to phosphorylate the receptor’s β and γ subunits and initiate signaling. The tyrosine kinase Syk is then recruited to the receptor’s phosphorylated γ chain, and this is followed by the assembly of a macromolecular signaling complex containing adaptor molecules (Grb2, LAT, Slp76) and enzymes (Vav, SOS) that ultimately lead to the endpoints of granule secretion, lipid metabolite generation, and synthesis of cytokines. One proposed pathway involves Syk-mediated phosphorylation of Btk, which activates phospholipase C (PLC). PLC in turn hydrolyzes phosphatidylinositol-bisphosphate (PIP2, found in the plasma membrane) to inositol 1,4,5-triphosphate (IP3) and 1,2-diacyl glycerol (DAG). IP3 functions by binding to receptors on the endoplasmic reticulum, mobilizing internal calcium stores, leading to a transient rise in the cytosolic concentration of calcium. Emptying of intracellular stores results in the opening of a plasma membrane channel (ICRAC), resulting in the sustained calcium flux needed for secretion. Both DAG and calcium can activate the protein kinase C (PKC) family of enzymes. PKC is able to phosphorylate many substrates including myosin light-chain kinase, which may act to change the cytoskeleton to permit degranulation. A separate pathway traced to the FcεRI complex activation involves the p21ras molecule leading to downstream activation of the mitogen-activated protein kinases (MAPK). The p21ras pathway is common to several receptors and involved in numerous cell functions including the activation of transcription factors that can enter the nucleus to activate cytokine genes. Elevations in intracellular calcium and phosphorylation of other MAPK
can activate cytoplasmic phospholipase A2 (cPLA2). cPLA2 cleaves membrane phospholipids to generate arachidonic acid and eventually leads to synthesis of prostaglandins (PGs) and leukotrienes (LTs).
can activate cytoplasmic phospholipase A2 (cPLA2). cPLA2 cleaves membrane phospholipids to generate arachidonic acid and eventually leads to synthesis of prostaglandins (PGs) and leukotrienes (LTs).
It is important to note that several agents can inhibit cellular degranulation, including ethylenediaminetetraacetic acid (by calcium chelation), cyclic adenosine monophosphate (cAMP), colchicine, cromolyn, beta-agonists, and corticosteroids (true for basophils but not mast cells). Therapeutic drugs that can block mediator release include those that target cAMP by either increasing its levels (e.g., beta-adrenergic agonists) or preventing the breakdown of cAMP by inhibiting phosphodiesterases (e.g., theophylline). Although it is known to inhibit degranulation, the specific intracellular targets of cromolyn are not understood. Some therapeutics that are under development or in early clinical testing specifically inhibit events early in the signaling cascade, such as the activation of Lyn or Syk tyrosine kinases.
E. Clinical significance
Mast cells. Mast cells serve as critical sensor cells between the environment and the host. They are initiator cells that release many mediators thought to be relevant in the pathogenesis of allergic diseases, including anaphylaxis, allergic rhinitis, and allergic asthma. Activating mutations in c-kit lead to mastocytosis, which is characterized by increased mast cell number and clinical features that resemble some of those in anaphylaxis. Evidence for mast cell involvement can be supported by the detection of elevated tryptase levels in serum. The monoclonal mast cell activation syndrome is characterized by aberrant, clonal mast cell populations.